Abstract
Many human gliomas carry markers characteristic of oligodendrocyte progenitor cells (such as Olig-2, PDGF alpha receptor and NG2 proteoglycan), suggesting these progenitors as the cells of origin for glioma initiation. This review considers the potential roles of the NG2 proteoglycan in glioma progression. NG2 is expressed not only by glioma cells and by oligodendrocyte progenitors, but also by pericytes associated with the tumor microvasculature. The proteoglycan may therefore promote tumor vascularization and recruitment of normal progenitors to the tumor mass, in addition to mediating expansion of the transformed cell population. Along with potentiating growth factor signaling and serving as a cell surface receptor for extracellular matrix components, NG2 also has the ability to mediate activation of β-1 integrins. These molecular interactions allow the proteoglycan to contribute to critical processes such as cell proliferation, cell motility and cell survival.
Key words: NG2 proteoglycan, glioma progression, cell motility, cell proliferation, cell survival, tumor vascularization
Introduction
In adults, diffusely infiltrating gliomas are by far the most commonly encountered type of primary intracranial tumor. Because of their sensitive location, these neoplasms present special therapeutic problems. Moreover, even after surgical removal, the aggressive nature of gliomas often leads to tumor recurrence and invasion into distant sites in the brain parenchyma. An additional problem is the frequent acquisition by gliomas of resistance to current chemo and radiation therapy regimens. This combination of properties renders gliomas extremely resistant to successful treatment.1,2 Improvements in glioma therapy will depend on increased understanding of several factors, including the origin of gliomas and molecular mechanisms responsible for their motility, proliferation, vascularization and resistance to induced cell death. At minimum, these pieces of knowledge will provide well-defined cellular and molecular targets for the design of new therapies.3
Although some gliomas may arise as a result of transforming events that affect mature oligodendrocytes and astrocytes, increasing attention is being paid to the idea that immature glial progenitor cells represent a more likely point of origin for these tumors. According to this idea, gliomas that arise during CNS development might stem from either astrocyte or oligodendrocyte progenitors. However, in adulthood oligodendrocyte progenitors represent the most abundant class of cycling progenitors4 and therefore appear to be the best candidates to serve as glioma stem cells. Accordingly, a high proportion of diffusely infiltrating human gliomas express markers that are characteristic of oligodendrocyte progenitors, including NG2 proteoglycan, PDGFα-receptor (PDGFRα) and Olig-2.5–7
This review will focus on the involvement of the NG2 proteoglycan in oligodendrocyte progenitor function and on the potential role of NG2 in glioma progression. The increasing availability of information concerning oligodendrocyte progenitor biology is likely to provide important insights into parallel aspects of glioma cell behavior. Both types of cells rely on cell motility and proliferation for success in their respective roles. In addition, both populations are subject to regulation by programmed cell death in order to control the size of the pool of cells. Before discussing possible ways that NG2 may influence glioma progression, it will be useful to review briefly some of the background concerning NG2 structure and expression. These topics will provide a context for understanding what is known about NG2 function as it may pertain to glioma progression.
NG2 Structure
NG2 is the rat homolog of the human melanoma proteoglycan8 and the mouse AN2 protein.9 NG2 is a type 1 transmembrane protein in which a 25-residue transmembrane domain separates a very large 2225 amino acid extracellular domain from a relatively short 76 amino acid cytoplasmic domain. Although no structural data for NG2 is available at the molecular level, some structural information can be inferred from the amino acid sequence10 and from biochemical studies of the molecule.
Extracellular Domain
Based on the amino acid sequence, predictions of secondary structure suggested that the NG2 ectodomain should contain two globular domains connected by a more extended central domain.11 Electron micrographs of rotary shadowed NG2 preparations reveal just such dumbbell-shaped structures.12 As shown in Figure 1, the amino-terminal one-third of the ectodomain, designated Domain1 (or D1), is stabilized by intramolecular disulfide bonding.10 This domain contains two laminin G-type motifs that might be important for ligand binding,13 although no specific information supports this possibility. The central D2 domain contains a single chondroitin sulfate chain at ser-999.14 The α helical amino terminal portion of domain 2 also contains the site for binding of collagens V and VI.11,12 The chondroitin sulfate chain has not been shown to be required for binding of these collagens or any other known NG2 ligand. Why NG2 exists as a proteoglycan is therefore somewhat of a mystery, although there is some evidence that the glycosaminoglycan chain is important for targeting the molecule to specific microdomains of the cell membrane.14 The globular D3 juxtamembrane one-third of the ectodomain contains N-linked oligosaccharides that are required for binding of galectin-3.15 Sites for binding of α1 integrins are also present in D3 (our unpublished data). D3 also contains sites for proteolysis of NG2, leading to its release from the cell surface.16,17 Proteolytic shedding of NG2 is greatly enhanced in several types of injuries.18–20 Although little is known about the mechanisms of shedding or the functions of shed NG2, it seems likely that this material can have important roles in a number of physiological processes.
Figure 1.
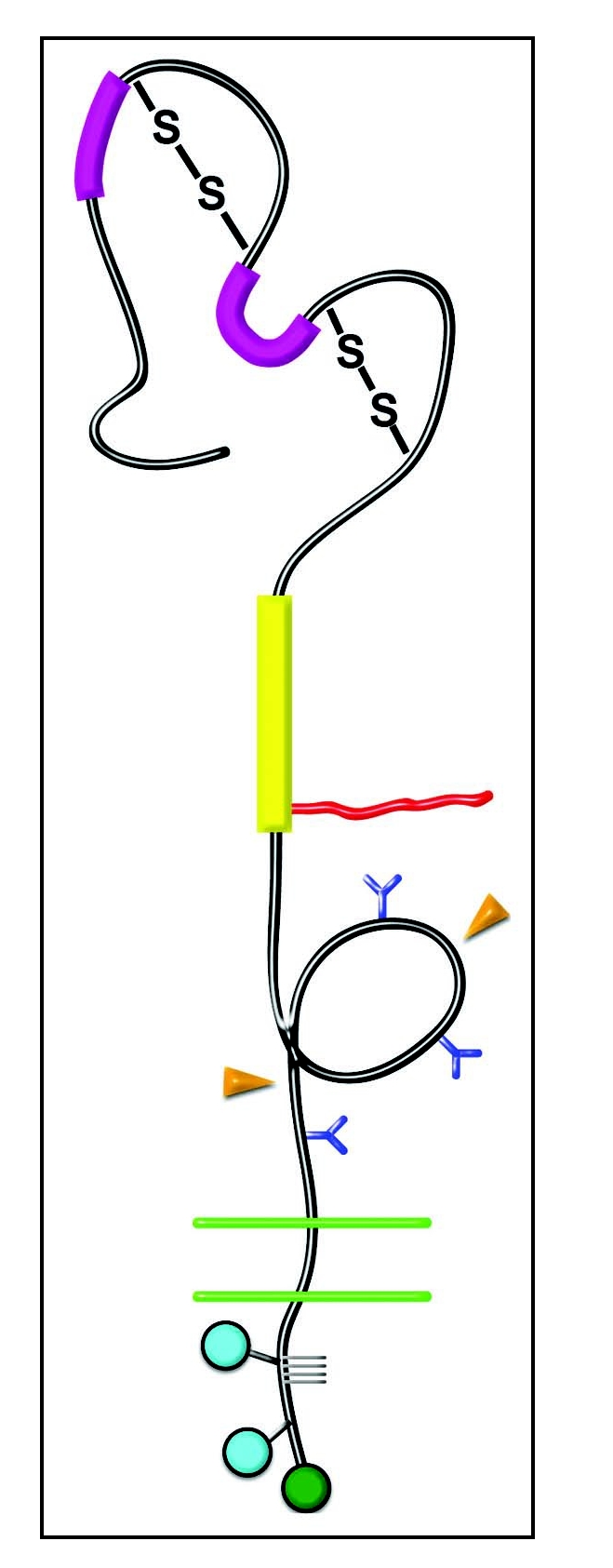
Domain Structure of NG2. Domain 1—Bold magenta bars, laminin G domains; S-S, disulfide bonds. Domain 2—Bold yellow bar, collagen binding domain; Irregular red line, chondroitin sulfate chain. Domain 3—Blue Y-shapes, N-linked oligosaccharides; Orange arrowheads, sites of proteolytic cleavage. Transmembrane Domain—Double green lines, plasma membrane. Cytoplasmic Domain—Blue circles, sites of threonine phosphorylation; Green circle, PDZ binding motif; Gray grid lines, proline-rich segment.
Cytoplasmic Domain
The NG2 cytoplasmic domain contains some recognizable motifs that are important for NG2 function. At the extreme C-terminus, the PDZ-binding motif QYWV mediates the interaction of NG2 with the multi-PDZ scaffolding proteins MUPP1,21 GRIP122 and syntenin-1.23 There are several cytoplasmic threonine residues, at least two of which are sites of functionally important phosphorylation. Thr-2256 is phosphorylated by PKCα,24 while Thr-2314 is phosphorylated by ERK.25 Although a classical PXXP SH3 binding domain is not present, the C-terminal half of the NG2 cytoplasmic tail is very rich in prolines. The significance of this remains to be established.
Patterns of NG2 Expression
The expression pattern of NG2 has always been one of its most interesting features. Long before there was much information concerning NG2 structure or function, the ability to use the proteoglycan as a marker for unique cell types made it a molecule of interest. A general rule of thumb concerning NG2 is that it is not expressed by multipotent stem cells, but is upregulated once stem cells make an initial commitment to a particular cellular lineage. NG2 is then strongly expressed on partially-committed progenitors that are still proliferative, motile and retain a certain degree of developmental plasticity. Upon terminal differentiation of these progenitors, NG2 expression is downregulated. Interestingly, NG2 is once again upregulated in many types of injury and pathological situations (including tumors) that are characterized by renewed cell proliferation and motility. Since NG2 expression is widespread in a number of developing tissues, these phenomena suggest that the proteoglycan is not so much a marker for a specific cell type, but is more of a marker for an “activated” (as opposed to quiescent) status of cells.
NG2 Expression Outside the Central Nervous System
Although NG2 was originally described in the context of the central nervous system, the proteoglycan has a widespread distribution in many other tissues. A brief description of some of these tissues will serve to illustrate the general trends mentioned in the foregoing paragraph. In the developing rodent limb, NG2 expression is not seen on undifferentiated stem cells of the early limb bud, but is upregulated in condensations of mesenchymal cells that represent immature chondroblasts.26,27 As chondroblasts differentiate into mature chondrocytes, NG2 expression is largely downregulated. A similar pattern of up and downregulation is observed during the osteoblast to osteocyte transition in maturing bone.27 In developing skin, NG2 is expressed by proliferating keratinocyte progenitor cells that are derived from slowly cycling, NG2-negative keratinocyte stem cells.28–30 NG2 expression is also prominent on progenitor cells associated with the hair follicle bulge region. As observed for many types of NG2-positive cells, bulge region progenitors give rise to a several types of differentiated progeny. NG2 is downregulated in mature cells derived from both keratinocyte and bulge region progenitors.
NG2 expression is also pronounced in developing vasculature. Despite some early confusion concerning the identity of NG2-positive vascular cell types, it is now clear that NG2 expression is restricted to vascular mural cells and is absent from the vascular endothelium. This is true in structures formed by both vasculogenic and angiogenic mechanisms. Thus, in the developing heart NG2 is found on cardiomyocytes, in macrovasculature it is found on smooth muscle cells, and in microvessels it is present on pericytes.31–35 The use of NG2 as a marker for nascent pericytes has led to the realization that pericytes play earlier and more important roles in vascularization than previously realized.36–40 As with progenitors from the hair follicle bulge region, the frequently-observed developmental plasticity of pericytes is one of the hallmarks of NG2-positive progenitor cells. While NG2 expression is downregulated to some extent in pericytes associated with quiescent vessels, it is highly upregulated again by pericytes in neovasculature associated with wound repair and other pathologies, including tumors. This will become important in our discussion of the possible role of NG2 in glioma progression.
NG2 Expression in the Central Nervous System
In the developing central nervous system, NG2 expression is largely restricted to oligodendrocyte progenitor cells (although NG2 is also highly expressed by pericytes associated with central nervous system vasculature). Along with PDGFRα, NG2 has become one of the most reliable markers for these progenitors.4,41–46 In keeping with general observations regarding NG2 expression, the proteoglycan is not expressed by multipotent neural stem cells in primary and secondary germinal zones of the central nervous system, but is upregulated on progenitors that emerge from these germinal zones and are committed to the oligodendrocyte lineage. Through a program of proliferation and migration, oligodendrocyte progenitors populate the entire central nervous system and differentiate into myelinating oligodendrocytes. This maturation is marked by downregulation of both NG2 and PDGFRα. Although differentiation of NG2-positive progenitors into astrocytes has been difficult to detect,47,48 the derivation of some grey matter astrocytes has now been convincingly documented from NG2-positive progenitors.49 This observation to some extent reconciles in vivo behavior with in vitro work showing that NG2-positive progenitors were capable of producing both oligodendrocytes and astrocytes, depending on the culture environment.50–52 These observations are in keeping with the developmental plasticity characteristic of NG2-positive progenitors.
Although central nervous system myelination in rodents is largely complete during the third and fourth weeks postnatally, large numbers of cycling NG2-positive and PDGFRα-positive cells persist in the adult rodent brain and spinal cord.4,53–55 This has led to much discussion as to whether these cells represent a large pool of persistent oligodendrocyte progenitors or a third class of differentiated macroglia designated as polydendrocytes56 or β neuroglial cells57 that are distinct from oligodendrocytes and astrocytes and have as yet unknown functions. The former opinion is supported by the observation that adult oligodendrocyte progenitors serve as the source of new oligodendrocytes for remyelination of demyelinated axons58,59 and that these progenitors proliferate in response to a wide variety of injuries to the central nervous system.46,60,61 The latter opinion is supported by the observations that adult polydendrocytes have differentiated morphologies distinct from those of simple neonatal progenitors,56 exhibit intimate spatial relationships with synaptic structures62 and nodes of Ranvier,63,64 and receive functional synaptic input from glutamatergic neurons.65–67 One possibility that may be consistent with both opinions is that the intimate relationships of NG2-positive glia with axons and synapses renders them extremely sensitive to changes in the neuronal environment, allowing them to respond to pathological challenges by generating additional populations of oligodendrocytes and/or polydendrocytes. Overall, it will not be surprising if NG2-positive glia/progenitors in the adult central nervous system prove to be a heterogeneous population of cells. From the standpoint of glioma development and progression, the key observation is that the adult central nervous system contains a large number of cycling NG2-positive progenitors/polydendrocytes that are potentially vulnerable to transformation.
NG2 Expression in Gliomas
Human gliomas have been proposed to originate from several types of neural cells, including astrocytes, stem cells and glial progenitors.68–71 The role of glial progenitors in this process is supported by the repeated observation that many gliomas express markers characteristic of oligodendrocyte progenitors. These markers include NG2, PDGFRα and Olig-2.5–7,72–74 Oligodendrocyte progenitors remain widespread in the adult brain42,56,75,76 and represent the most abundant population of cycling cells in the adult central nervous system,4,53,55,77 thus providing a likely pool of cells in which accumulating mutations can lead to gliomagenesis. The sensitivity of these progenitors to mitogenic stimulation may play a role in their susceptibility to transformation. Indeed, transformation via overexpression of PDGF provides the basis for a number of commonly used rodent glioma models.3,71,78–80 In the case of NG2, several reports suggest that expression of the proteoglycan is correlated with the degree of malignancy of the glioma.72,73,81–84 This would be consistent with our ideas concerning the ability NG2 to potentiate cell motility and cell proliferation in response to stimulation by growth factors and extracellular matrix components. This will be discussed more fully in a later section.
In a most revealing recent study, retroviral overexpression of PDGF-B was used to drive gliomagenesis in adult rat white matter,85 a paradigm that is highly relevant to the observation that human gliomas originate largely in the subcortical white matter of the adult central nervous system. The resulting tumors were highly invasive and, like human glioblastoma multiforme, were characterized by extensive vascularization and pseudopallisading necrosis. As in the examples cited in foregoing paragraphs, these tumors were composed largely of cells that expressed the oligodendrocyte progenitor markers NG2, PDGFRα and Olig-2, indicating their origin from cycling adult white matter progenitors. Unexpectedly, however, only 20% of the tumor-associated cells were actually infected by the PDGF-B retrovirus (as determined via a vector-encoded GFP reporter). The other 80% of the tumor mass was composed of apparently normal oligodendrocyte progenitors recruited via growth factors produced by the transformed cell population.85 Recruitment of this sort is also observed in human gliomas.80,86,87 Molecules such as NG2 and PDGFRα that are important for the proliferation and motility of oligodendrocyte progenitors would be expected to be critical for this recruitment to the glioma mass.
NG2 Expression in Tumor Vasculature
As in normal blood vessels, NG2 is expressed by pericytes in tumor microvasculature.33,38,88,89 It is often noted that pericyte-endothelial cell relationships are disrupted in tumor vessels.36,37,90 We have reported the frequent occurrence in tumors of NG2-positive “pericyte tubes,” segments of blood vessels that are devoid of endothelial cells.38 This reflects not only the early role of pericytes in vessel formation, but also the dis-regulation of pericyte-endothelial cell interaction under pathological conditions.
Since gliomas are characterized by aggressive vascularization,91 the expression and role of NG2 in glioma vasculature is an important topic. Robust expression of NG2 in glioma vasculature has been reported by several groups.32,72,82,92 Intriguingly, expression of NG2 by glioma cells themselves has also been noted to have important effects on the characteristics of the glioma vasculature.81,93
NG2 Function
Very early in our work, the expression pattern of NG2 on immature progenitor cells suggested to us that the proteoglycan might contribute to processes such as cell proliferation and motility that are critical to progenitor biology. This impression was strengthened by the finding that NG2 is often re-expressed by tumor cells, which are usually characterized by increased proliferation and migration. Indeed we have shown that expression of NG2 increases the tumorigenic and metastatic properties of mouse melanoma cells.94 In addition to its wide expression on melanomas,95 NG2 is also found on glioblastomas,5,72–74,83 chondrosarcomas96 and lymphoid leukemias.97 Subsequent studies in our lab and others have tried to identify mechanisms by which NG2 might influence various aspects of cell behavior including proliferation and migration. A large part of this research has been concerned with defining the interactions of NG2 with extracellular and intracellular binding partners in order to establish the molecular basis for an interface between NG2 and cytoplasmic signaling processes.
As a general rule it has been somewhat difficult to assign specific functions to proteoglycans, since they do not usually have enzymatic activities or dramatic signal transducing properties. In the case of glycosaminoglycan-rich proteoglycans, conventional wisdom suggests that these molecules serve space-filling functions based on their high degree of hydration. However, this does not apply to the case of NG2 and many other proteoglycans that have only one or relatively few glycosaminoglycan chains. In many cases such proteoglycans appear to serve accessory functions, in which they potentiate or regulate the activities of more well-known signal transducing systems, such as integrins or growth factor receptors. John Couchman has referred to the syndecan family of heparan sulfate proteoglycans as regulators of cell surface microdomains.98 Even though NG2 is a chondroitin sulfate proteoglycan, this also appears to be a very apt description of its mode of action. Although NG2 does seem to have some modest signal transducing capabilities of its own,99–101 this is greatly outweighed by its ability to potentiate the activities of β1 integrins and growth factor receptors. In the following sections, we will discuss these activities of NG2 in the context of mechanisms that are important in the biology of both oligodendrocyte progenitors and gliomas. In light of the apparent derivation of gliomas from oligodendrocyte progenitors, clues from progenitor biology may provide important insights into parallel aspects of glioma cell behavior.
Cell Motility
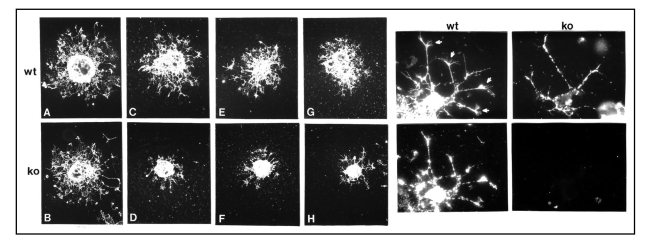
Just as cell motility is a critical aspect of the ability of oligodendrocyte progenitors to spread throughout the developing central nervous system, it is also important for the ability of gliomas to aggressively invade new areas in the brain. Several pieces of information suggest that NG2 plays a role in effective cell migration. Early work on NG2 showed that melanoma cell attachment and spreading could be inhibited by NG2 antibodies,102,103 and that NG2 was capable of triggering rearrangement of the actin cytoskeleton.99,104,105 The first demonstration that NG2 can be important for cell motility came as a result of the finding that NG2 is a cell surface ligand for type VI collagen.11,12,101,106–108 Collagen VI binds to the extended central D2 domain of NG2, as shown not only by solid phase binding assays with purified NG2 fragments,12 but also by studies in which recombinant deletion mutants of NG2 were expressed in rat B28 glioma cells.11 NG2 transfectants lacking the D2 domain showed unchanged motility in the presence of collagen VI, whereas full-length transfectants and transfectants that included D2 exhibited increased motility when exposed to collagen VI (compared to parental B28 cells). Similar results were also obtained with human U251 glioma cells transfected with NG2.11 Moreover, oligodendrocyte progenitors from wild type mice migrate very well on collagen VI-coated surfaces. In contrast, progenitors from NG2 null mice are much less motile on these same surfaces, emphasizing the importance of NG2 for cell motility in response to collagen VI (Fig. 2). Comparable results have been achieved on laminin 2-coated surfaces. The significance of these experiments may be questioned on the grounds that collagen VI and laminin-2 are not major components of the brain parenchyma. However, their association with brain vasculature and with axonal processes could provide a means for migration of NG2-positive glioma cells along blood vessels and nerve fiber tracts.109–111
Figure 2.
Role of NG2 in cell motility. Left, Oligodendrocyte progenitor motility. Oligodendrocyte progenitors prepared from neonatal wild type (wt) and NG2 knockout (ko) brains were grown as aggregates and then plated on surfaces coated with fibronectin (A and B) or type VI collagen (C–H). After 36 hours, cultures were labeled with A2B5 antibody to visualize progenitor migration away from the body of the aggregate. Although wild type and knockout progenitors migrate equally well on fibronectin-coated surfaces, NG2 null progenitors migrate much more poorly on collagen VI due to the absence of NG2. Right, Process extension by oligodendrocyte progenitors. Wild type (wt) and NG2 null (ko) oligodendrocyte progenitors were plated on type VI collagen coated surfaces. After 36 hours, cells were labeled with A2B5 antibody (top) or with NG2 antibody (bottom). Bottom panels confirm that NG2 is absent from NG2 null progenitors. Top panels show that processes of wild type progenitors are tipped by complex growth cone-like structures (arrows). These structures are absent from the processes of NG2 null progenitors. Growth cone-like structures may be important for mediating the motility of wild type progenitors on collagen VI, as seen in the left-hand panels.
Exposure to NG2 itself was found to stimulate motility in vascular endothelial cells, which do not themselves express the proteoglycan. This trans effect of NG2 was shown to be due to interaction of the proteoglycan with a galectin-3/α3β1 integrin complex on the endothelial cell surface, resulting in enhanced β1 integrin signaling, greater endothelial cell motility, enhanced endothelial tube formation in vitro, and dramatically increased blood vessel development in vivo.112 These phenomena are significant because of the intimate interaction that exists in blood vessels between endothelial cells and NG2-positive pericytes. NG2 may be important as one element of the crosstalk that occurs between endothelial cells and pericytes. The significance of this will become more apparent when we discuss neovascularization.
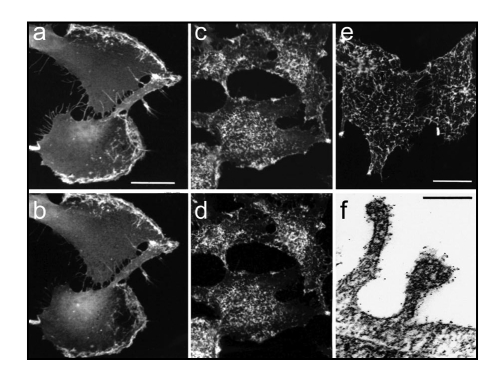
Since NG2 has previously been implicated as a co-receptor for β1 integrin ligands113–115 and since we were aware of several tumor cell types in which NG2 and α3β1 integrin are co-expressed and form a physical complex on the cell surface, we wondered if NG2-dependent α3β1 integrin activation could also occur via a cis mechanism. To examine this possibility, we compared the motility of parental U251 glioma cells and NG2-transfected U251 cells. Under basal conditions, the motilities of U251 and U251/NG2 cells were similar. Upon stimulation with PMA or PDGF, however, U251/NG2 motility increased significantly compared to that of U251 cells. Further investigation showed that both PMA and PDGF triggered PKCα-dependent phosphorylation of NG2 at Thr-2256,24,25 and that this phosphorylation event was required for the increase in motility. A Thr-2256-Val NG2 mutant was incapable of increased motility, while a Thr-2256-Glu mutant was spontaneously motile even in the absence of PMA or PDGF. Increased β1 integrin activation (assessed by binding of the activation-dependent HUTS-21 antibody116) was detected in the Thr-2256-Glu mutant as well as in PMA or PDGF-treated U251/NG2 cells. Intriguingly, NG2 phosphorylated at Thr-2256 was found to be co-localized with α3β1 integrin in broad lamellipodia at the leading edges of motile cells (Fig. 3). This represents a translocation of NG2 from its co-localization with α3β1 integrin on apical microprojections in non-motile cells, suggesting that NG2 phosphorylation at Thr-2256 is responsible for relocation of the NG2/integrin complex to lamellipodia, accompanied by increased cell motility.
Figure 3.
NG2 co-localization with α-1 integin. (a and b) NG2-transfected U251 glioma cells were treated for three hours with PMA, fixed with 4% paraformaldehyde, and double stained with antibodies against NG2 (a) and β-1 integrin (b). NG2 and the integrin are co-localized to broad lamellipodia on the leading edges of cells. (c and d) Quiescent NG2-transfected U251 cells were fixed with 4% paraformaldehyde and double-stained with antibodies against NG2 and β-1 integrin (d). NG2 and the integrin are co-localized to arrays of microprotrusions on the apical cell surface. (e) Quiescent NG2-transfected U251 cells were stained live at 4°C with NG2 antibody to confirm the cell surface (as opposed to cytoplasmic) location of apical microprotrusions. (f) Immunogold electron microscopy of NG2 labeling reveals that NG2 (black puncta) is heavily localized to microprotrusions from the apical cell surface.
Cell Proliferation
In addition to its role in expansion of the oligodendrocyte progenitor population during central nervous system development, cell proliferation makes an obvious contribution to glioma growth and progression. A key finding with regard to the role of NG2 in cell proliferation was that the proteoglycan is capable of binding with high affinity to the growth factors FGF2 and PDGF-AA.117 Heparan sulfate proteoglycans are well-known for their ability to serve as co-receptors for FGF family members due to FGF affinity for heparan sulfate.118 However, in the case of NG2, the core protein rather than the chondroitin sulfate chain is responsible for growth factor binding, with putative binding sites scattered throughout the D2 and D3 domains.
Since both FGF2 and PDGF-AA are critical for expansion of the oligodendrocyte progenitor population, it has been tempting to suppose that NG2 could be important for progenitor responsiveness to these two factors. An initial result supporting this idea was that treatment with anti-NG2 antibody inhibited proliferation of oligodendrocyte progenitors.43 Subsequently, we showed that, whereas the combined action of FGF2 and PDGF-AA was able to maintain wild type progenitors in their undifferentiated state, NG2 null progenitors began the process of differentiation even in the presence of the two growth factors.119 More careful work done with aortic smooth muscle cells showed that NG2 null cells failed to proliferate or migrate normally in response to PDGF-AA, due to poor activation of PDGFRα.120 In contrast, receptor activation was robust in wild type cells, accompanied by good mitogenic and motility responses.
A similar set of findings using smooth muscle cell lines has been made by Dr. Roberto Perris' lab in the case of FGF2. In the absence of NG2, cells do not sequester FGF2 at the cell surface and therefore are unable to activate FGF receptors (unpublished results), leading to low proliferative responses. An indication that this phenomenon also occurs in vivo comes from a corneal angiogenesis model in which an FGF2-containing pellet is used to stimulate blood vessel growth into the corneas of wild type and NG2 null mice.121 Responsiveness to FGF2 is excellent in wild type corneas, but is reduced by at least four-fold in NG2 null corneas. This appears to be largely a result of poor pericyte proliferation in the absence of NG2. The use of BrdU incorporation to label mitotic cells shows that proliferation of NG2 null pericytes is reduced by a factor of three compared to wild type pericytes, presumably due to lack of responsiveness to FGF2.
Phosphorylation of NG2 also plays a role in cell proliferation. Whereas PKCα-mediates phosphorylation of NG2 at Thr-2256, leading to enhanced motility, ERK catalyzes phosphorylation of NG2 at Thr-2314, stimulating cell proliferation.25 Under non-stimulatory conditions, NG2-transfected U251 glioma cells are more proliferative than parental U251 cells due to basal levels of NG2 phosphorylation at Thr-2314. Thr-2314-Glu mutants exhibit even higher rates of proliferation, while Thr-2314-Val mutants are indistinguishable from non-transfected U251 cells. Interestingly, α3β1 integrin activation is also required for this NG2-dependent increase in proliferation, begging the question how NG2-stimulated integrin activation promotes motility in one case and proliferation in another. The answer seems to lie in the localization of the NG2/integrin complex to two distinct microdomains, depending on the NG2 phosphorylation status. NG2 phosphorylated at Thr-2314 is co-localized with α3β1 integrin on microprojections on the apical cell surface. NG2 phosphorylated at Thr-2256 is co-localized with α3β1 integrin in leading edge lamellipodia. The site of integrin activation is therefore different in the two cases. In lamellipodia the integrin presumably interacts preferentially with cytoplasmic machinery required for motility. In apical microprojections the integrin must interact with a different set of signaling molecules required for proliferation. NG2 is therefore able to help strike a balance between cell proliferation and cell motility, depending on incoming signaling that determines the NG2 phosphorylation state. This implies that the site of cytoplasmic phosphorylation of NG2 can determine its ability to interact with cytoplasmic scaffolding proteins that anchor the proteoglycan (and its integrin binding partner) within specific membrane microdomains. MUPP1,21 GRIP1,22 syntenin-123 and ezrin24 are possible candidates for such anchoring functions. In this respect, syntenin-1 has been implicated in NG2-dependent oligodendrocyte progenitor migration.23
Cell Survival
Chemoresistance is an important and problematic characteristic of many gliomas. Interestingly, in addition to its effects on cell proliferation and migration, NG2-dependent activation of α3β1 integrin also has effects on cell survival due to increased signaling through the PI3K/AKT pathway.122,123 NG2-transfected U251 glioma cells are resistant to treatment with TNFα and chemotherapeutic drugs such as doxorubicin, vincristine and etoposide that effectively trigger apoptosis in parental U251 cells.84 siRNA-mediated knockdown of NG2 expression effectively restores apoptosis sensitivity in U251/NG2 cells, further demonstrating the cause and effect relationship between NG2 expression and apoptosis resistance. NG2 knockdown is also effective in increasing apoptosis sensitivity in endogenous NG2 expressing glioma lines such as U87 and A172, as well as in the A375 melanoma line, demonstrating that NG2-dependent apoptosis resistance is a widespread phenomenon in several tumor types. In all cases, there was a direct correlation of NG2 expression level with both β1 integrin activation and the level of AKT phosphorylation. Two types of evidence indicate that the phenomenon operates in vivo as well as in vitro. First, U87 cells produce significantly larger, faster growing tumors in NOD-SCID mice than U87 cells treated with NG2 siRNA to knock down expression of the proteoglycan in vivo. Growth of the siRNA-treated tumors is further inhibited by administration of TNFα, a phenomenon not seen in U87 tumors without siRNA treatment. This demonstrates the increased apoptosis sensitivity provided by NG2 knockdown. The TNFα-independent decrease in tumor growth provided by NG2 knockdown is likely due to the effects of NG2 on other parameters such as cell proliferation, reinforcing the idea that NG2 affects multiple aspects of glioma progression. Second, human glioma biopsy samples grown in spheroid cultures were tested for sensitivity to the chemotherapy drugs doxorubicin, etoposide and carboplatin. There was an excellent correlation between apoptosis resistance and the level of NG2 expression in these tumor samples.84
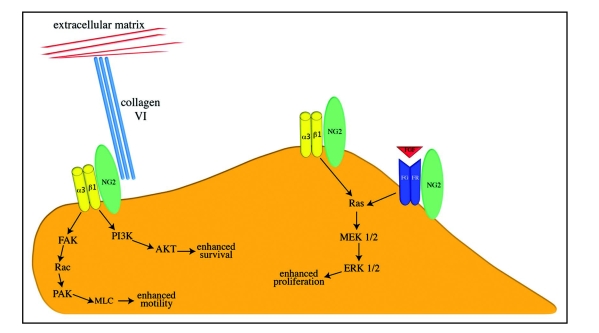
The cartoon in Figure 4 summarizes our ideas concerning the functional effects of NG2 on cell proliferation, migration, survival and interaction with the extracellular matrix. For the sake of simplicity, the signaling pathways thought to be involved in these phenomena are shown in abbreviated form without any attempt to capture the complete mechanisms.
Figure 4.
Functional Interactions of NG2. On the apical cell surface, the thr-2314 phosphorylated NG2 proteoglycan (green oval) activates α3β1 integrin (yellow dimer) signaling to promote enhanced proliferation. NG2 also promotes cell proliferation via potentiation of growth factor/growth factor receptor signaling (indicated here by red FGF and blue FGFR dimer). On leading edge lamellipodia, thr-2256 phosphorylated NG2 activates α3β1 integrin signaling to promote enhanced motility. NG2-mediated integrin signaling can also enhance cell survival via the PI3/AKT pathway. NG2 also provides a linkage between the cell surface and the extracellular matrix via its interaction with type VI collagen (triple turqouise rods). Signaling pathways are shown in abbreviated format due to space considerations.
Neovascularization
Through the work of Judah Folkman and others,124 it is well accepted that tumors require effective vascularization for their growth and expansion. Since highly invasive gliomas are characterized by their extensive vascularization, the role of NG2 in blood vessel development is extremely relevant to glioma progression. While the majority of vascularization studies focus on the endothelial component of blood vessels, it is now clear that pericytes are also an early and critical component of microvascular development. In some types of neovascularization, pericytes are among the first cell types observed in nascent blood vessels, sometimes even preceding the arrival of endothelial cells.38–40,92,125,126 The best evidence for the importance of pericytes in vascularization is the impaired pericyte development and resulting aberrant vasculature produced by genetic ablation of PDGF-B or PDGFRβ.37,127–129
NG2 also has an important role in pericyte development and function, as demonstrated by the decreased postnatal neovascularization observed in the NG2 null mouse. In ischemic retinal vascularization and in corneal vascularization induced by FGF2, blood vessel development is decreased more than 2-fold by genetic ablation of NG2.121 The NG2-deficient vasculature that forms in these pathological eye models is characterized by a diminished pericyte:endothelial cell ratio (dropping from 1:1 to as low as 1:4). The most obvious cause of this change is the reduced proliferation of pericytes in the absence of NG2, as detected by BrdU incorporation.121 It is possible that reduced motility of pericytes also contributes to the decrease in pericyte number, but this has not yet been specifically addressed. Another topic of future interest is the effect of NG2 ablation on endothelial cells. In addition to the reduced proliferation of pericytes observed in the ischemic retinal model, we have also observed (although to a lesser degree) reduced endothelial cell proliferation. We have speculated that this might be due to the ability of pericyte-derived NG2 to mediate recruitment of endothelial cells.112 Another possibility is suggested by the finding that NG2 binds to the kringle domains of angiostatin and blocks the ability of angiostatin to inhibit endothelial cell proliferation.81,130 In both of these scenarios, the absence of NG2 would be expected to diminish endothelial cell recruitment and/or proliferation.
It is our expectation that the role of NG2 in promoting pericyte development and neovascularization will also be evident during tumor growth. Unpublished data from our lab indicate that mammary tumors in the MMTV-PyMT transgenic mouse model131,132 develop more slowly and with reduced metastasis on an NG2 null background. Since NG2 is not expressed by the mammary tumor cells in this model, the effect of NG2 ablation must be on an element (or elements) of the tumor stroma. Preliminary tests indicate that blood vessel development is altered in the NG2 null tumors. Compared to blood vessels in wild type tumors, vessels in NG2 null tumors exhibit reduced diameters and diminished investment by pericytes.
Glioma Models for Assessing NG2 Function
We have made preliminary observations of NG2 contributions to glioma progression in two different animal models. In the first paradigm, we have collaborated with Peter Canoll and Marcela Assanah to induce glioma formation via injection of a PDGF-B expressing retrovirus85 into the white matter of adult wild type C57Bl/6 mice and NG2 null C57Bl/6 mice. Table 1 shows that 4 of 11 wild type mice in this study developed lethal gliomas, while only 1 of 12 NG2 null mice developed a such a tumor. Clearly, these numbers are too small to be taken as significant, and more work needs to be done to render the model sufficiently robust to be useful. Nevertheless, the trend is very suggestive of an important effect of NG2 in promoting glioma progression. As discussed in previous sections, NG2 might be involved at multiple levels of tumor development. As the most abundant cycling cells in the white matter, oligodendrocyte progenitors are the principal cell type transformed by the virus. In the absence of NG2, decreased progenitor cycling may reduce the frequency of viral transformation events. The absence of NG2 may also decrease the proliferation and/or migration of transformed progenitors, along with recruitment of normal progenitors to the tumor mass, leading to reduced tumor progression. Finally, the absence of NG2 may reduce tumor vascularization via effects on pericyte recruitment.
Table 1.
Glioma models of NG2 function
| in vivo PDGF-B transformation tumor latency (months) | CT2A tumors tumor volume (mm3) | ||
| wt (11 mice total) | ko (12 mice total) | wt (5 mice total) | ko (5 mice total) |
| 4 | 11 | 330 | 50 |
| 6 | 280 | 20 | |
| 8 | 270 | 20 | |
| 12 | 175 | 10 | |
| 50 | 10 | ||
| Ave 220 | 20 | ||
In vivo PDGF-B transformation. PDGF-B retrovirus was microinjected into the subcortical white matter of 11 adult wild type (wt) and 12 adult NG2 null (ko) mice. Tumor latency is defined as the survival time of the mice. 4 of 11 wt mice produced gliomas, compared with 1 of 12 ko mice. CT2A tumors. CT2A astrocytoma cells (2 × 104 in 1 µl) were microinjected into the subcortical white matter of 5 adult wt and 5 adult NG2 ko mice. After three weeks, mice were perfused with paraformaldehyde and tumor volumes were estimated from tumor dimensions determined by dissection.
The second paradigm has utilized microinjection of CT2A mouse glioma cells133 into the white matter of five adult wild type and five adult NG2 null C57Bl/6 mice. Table 1 shows a dramatic difference in the size of the tumors that develop in the two mouse lines after three weeks. Tumors in wild type mice are on average 10-fold larger than tumors in NG2 null mice. Since CT2A cells express NG2,134 the differential effects of NG2 on tumor progression in wild type and NG2 null mice must come from elements of the host stroma, possibly including pericytes in the tumor vasculature and oligodendrocyte progenitors recruited to the tumor mass. Exploitation of cre/lox technology to produce cell type-specific ablation of NG2 should be useful in sorting out these issues. We anticipate that further refinements of these two glioma models, along with detailed analysis of the resulting tumors, will allow us to make a number of important advances in understanding the contributions of NG2 to glioma progression.
Acknowledgements
Work in our laboratory has been supported by NIH grants R01 CA95287 and P01 HD25938, and by California Tobacco Related Disease Research Program grant 15RT-0034.
Abbreviations
- ERK
extracellular signal-related kinase
- GRIP1
glutamate receptor interacting protein 1
- MUPP1
multi-PDZ domain protein 1
- NG2
nerve-glial antigen 2
- PDGF
platelet-derived growth factor
- PI3K
phosphoinositol-3 kinase
- PKC
protein kinase C
- PMA
phorbol-12-myristate-13-acetate
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/6279
References
- 1.Holland E. Glioblastoma multiforme: the terminator. Proc Natl Acad Sci USA. 2000;97:6242–6244. doi: 10.1073/pnas.97.12.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giese A, Bjerkvig R, Berens M, Westphal M. Cost of migration: invasion of malignant gliomas and Implications for treatment. J Clin Oncol. 2003;21:1624–1636. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 3.Hu X, Holland E. Applications of mouse glioma models in preclinical trials. Mut Res. 2005;576:54–65. doi: 10.1016/j.mrfmmm.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 4.Dawson M, Polito A, Levine J, Reynolds R. NG2 expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 5.Chekenya M, Pilkington G. NG2 precursor cells in neoplasia: functional, histogenesis and therapeutic implications for malignant brain tumours. J Neurocytol. 2002;31:507–521. doi: 10.1023/a:1025795715377. [DOI] [PubMed] [Google Scholar]
- 6.Bouvier C, Bartloi C, Aguirre-Cruz L, Virard I, Colin C, Fernandez C, Gouvernet J, Figarella-Branger D. Shared oligodendrocyte lineage gene expression in gliomas and oligodendrocyte progenitor cells. J Neurosurg. 2003;99:344–350. doi: 10.3171/jns.2003.99.2.0344. [DOI] [PubMed] [Google Scholar]
- 7.Ligon K, Alberta J, Kho A, Weiss J, Kwaan M, Nutt C, Louis D, Sties C, Rowitch D. The oligodendroglial lineage marker Olig2 is universally expressed in diffuse gliomas. J Neuropathol Exp Neurol. 2004;63:499–509. doi: 10.1093/jnen/63.5.499. [DOI] [PubMed] [Google Scholar]
- 8.Pluschke G, Vanek M, Evans A, Dittmar T, Schmid P, Itin P, Filardo E, Reisfeld R. Molecular cloning of a human melanoma-associated chondroitin sulfate proteoglycan. Proc Natl Acad Sci USA. 1996;93:9710–9715. doi: 10.1073/pnas.93.18.9710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider S, Bosse F, D'Urso D, Muller H, Sereda M, Nave K, Niehaus A, Kempf T, Schnolzer M, Trotter J. The AN2 protein is a novel marker for the Schwann cell lineage expressed by immature and nonmyelinating Schwann cells. J Neurosci. 2001;21:920–933. doi: 10.1523/JNEUROSCI.21-03-00920.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishiyama A, Dahlin K, Prince J, Johnstone S, Stallcup W. The primary structure of NG2, a novel membrane-spanning proteoglycan. J Cell Biol. 1991;114:359–371. doi: 10.1083/jcb.114.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burg M, Nishiyama A, Stallcup W. A central segment of the NG2 proteoglycan is critical for the ability of glioma cells to bind and migrate toward type VI collagen. Exp Cell Res. 1997;235:254–264. doi: 10.1006/excr.1997.3674. [DOI] [PubMed] [Google Scholar]
- 12.Tillet E, Ruggiero F, Nishiyama A, Stallcup W. The membrane-spanning proteoglycan NG2 binds to collagens V and VI through the central non-globular domain of its core protein. J Biol Chem. 1997;272:10769–10776. doi: 10.1074/jbc.272.16.10769. [DOI] [PubMed] [Google Scholar]
- 13.Stegmuller J, Schneider S, Hellwig A, Garwood J, Trotter J. AN2, the mouse homolog of NG2, is a surface antigen on glial precursor cells implicated in control of cell migration. J Neurocytol. 2002;31:497–505. doi: 10.1023/a:1025743731306. [DOI] [PubMed] [Google Scholar]
- 14.Stallcup W, Dahlin-Huppe K. Chondroitin sulfate and cytoplasmic domain-dependent membrane targeting of the NG2 proteoglycan propmotse retraction fiber formation and cell polarization. J Cell Sci. 2001;114:2315–2325. doi: 10.1242/jcs.114.12.2315. [DOI] [PubMed] [Google Scholar]
- 15.Wen Y, Makagiansar I, Fukushi J, Liu FT, Fukuda MN, Stallcup W. Molecular basis of interaction between NG2 proteoglycan and galectin-3. J Cell Biochem. 2006;98:115–127. doi: 10.1002/jcb.20768. [DOI] [PubMed] [Google Scholar]
- 16.Nishiyama A, Lin X, Stallcup W. Generation of truncated forms of the NG2 proteoglycan by cell surface proteolysis. Mol Biol Cell. 1995;6:1819–1832. doi: 10.1091/mbc.6.12.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asher R, Morgenstern D, Properzi F, Nishiyama A, Levine M, Fawcett J. Two separate metalloproteinase activities are responsible for the shedding and processing of the NG2 proteoglycan in vitro. Mol Cell Neurosci. 2005;29:82–96. doi: 10.1016/j.mcn.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Jones L, Yamaguchi Y, Stallcup W, Tuszynski M. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci. 2002;22:2792–2803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsen P, Wells J, Stallcup W, Opdenakker G, Yong V. Matrix metalloproteinase-9 facilitates remyelination in part by processing the inhibitory NG2 proteoglycan. J Neurosci. 2003;23:11127–11135. doi: 10.1523/JNEUROSCI.23-35-11127.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Castro R, Tajrishi R, Claros J, Stallcup W. Differential responses of spinal axons to transection: influence of the NG2 proteoglycan. Exp Neurol. 2005;192:299–309. doi: 10.1016/j.expneurol.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 21.Barritt D, Pearn M, Zisch A, Lee S, Javier R, Pasquale E, Stallcup W. The multi-PDZ protein MUPP1 is a cytoplasmic ligand for the membrane-spanning proteoglycan NG2. J Cell Biochem. 2000;79:213–224. doi: 10.1002/1097-4644(20001101)79:2<213::aid-jcb50>3.0.co;2-g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stegmuller J, Werner H, Nave K, Trotter J. The proteoglycan NG2 is complexed with AMPA receptors by the PDZ glutamate receptor interaction protein (GRIP) in glial progenitor cells: implications for glial-neuronal signaling. J Biol Chem. 2003;278:3590–3598. doi: 10.1074/jbc.M210010200. [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee N, Stegmuller J, Schatzle P, Karram K, Koroll M, Werner H, Nave K, Trotter J. Interaction of syntenin-1 and the NG2 proteoglycan in migratory oligodendrocyte precursor cells. J Biol Chem. 2008;283:8310–8317. doi: 10.1074/jbc.M706074200. [DOI] [PubMed] [Google Scholar]
- 24.Makagiansar I, Williams S, Dahlin-Huppe K, Fukushi J, Mustelin T, Stallcup W. Phosphorylation of NG2 proteoglycan by protein kinase C-α regulates polarized membrane distribution and cell motility. J Biol Chem. 2004;279:55262–55270. doi: 10.1074/jbc.M411045200. [DOI] [PubMed] [Google Scholar]
- 25.Makagiansar I, Williams S, Mustelin T, Stallcup W. Differential phosphorylation of NG2 proteoglycan by ERK and PKCα helps balance cell proliferation and migration. J Cell Biol. 2007;178:155–165. doi: 10.1083/jcb.200612084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishiyama A, Dahlin K, Stallcup W. The expression of NG2 proteoglycan in developing rat limb. Development. 1991;111:933–944. doi: 10.1242/dev.111.4.933. [DOI] [PubMed] [Google Scholar]
- 27.Fukushi J, Inatani M, Yamaguchi Y, Stallcup W. Expression of NG2 proteoglycan during endochondral and intramembranous ossification. Devel Dyn. 2003;228:143–148. doi: 10.1002/dvdy.10359. [DOI] [PubMed] [Google Scholar]
- 28.Legg J, Jensen U, Broad S, Leigh I, Watt F. Role of melanoma chondroitin sulfate proteoglycan in patterning stem cells in human interfollicular epidermis. Development. 2003;130:6049–6063. doi: 10.1242/dev.00837. [DOI] [PubMed] [Google Scholar]
- 29.Ghali L, Wong S, Tidman N, Quinn A, Philpott M, Leigh I. Epidermal and hair follicle progenitor cells express melanoma-associated proteoglycan core protein. J Invest Dermatol. 2004;122:433–442. doi: 10.1046/j.0022-202X.2004.22207.x. [DOI] [PubMed] [Google Scholar]
- 30.Kadoya K, Fukushi J, Matsumoto Y, Yamaguchi Y, Stallcup W. NG2 proteoglycan expression in mouse skin: altered postnatal skin development in the NG2 null mouse. J Histochem Cytochem. 2008;56:295–303. doi: 10.1369/jhc.7A7349.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grako K, Stallcup W. Participation of the NG2 proteoglycan in rat aortic smooth muscle cell responses to PDGF. Exp Cell Res. 1995;22:231–240. doi: 10.1006/excr.1995.1371. [DOI] [PubMed] [Google Scholar]
- 32.Schlingemann R, Rietvold F, De Wall K, Ferrone S, Ruiter D. Expression of the high molecular weight melanoma associated antigen by pericytes during angiogenesis in tumors and in healing wound. Am J Pathol. 1990;136:1393–1405. [PMC free article] [PubMed] [Google Scholar]
- 33.Burg M, Pasqualini R, Arap W, Ruoslahti E, Stallcup W. NG2 proteoglycan-binding peptides target tumor neovasculature. Cancer Res. 1999;59:2869–2874. [PubMed] [Google Scholar]
- 34.Ozerdem U, Grako K, Dahlin-Huppe K, Monosov E, Stallcup W. The NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222:218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- 35.Ozerdem U, Monosov E, Stallcup W. NG2 proteoglycan expression by pericytes in pathological microvasculature. Microvasc Res. 2002;63:129–134. doi: 10.1006/mvre.2001.2376. [DOI] [PubMed] [Google Scholar]
- 36.Bergers G, Song S. The role of pericytes in blood vessel formation. Neurooncology. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Betsholtz C, Lindblom P, Gerhardt H. Role of pericytes in vascular morphogenesis. EXS. 2005;94:115–125. doi: 10.1007/3-7643-7311-3_8. [DOI] [PubMed] [Google Scholar]
- 38.Ozerdem U, Stallcup W. Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis. 2003;6:241–249. doi: 10.1023/B:AGEN.0000021401.58039.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Virgintino D, Girolamo F, Errede M, Capobianco C, Robertson D, Stallcup W, Perris R, Roncali L. An intimate interplay between precocious migrating pericytes and endothelial cells governs human fetal brain angiogenesis. Angiogenesis. 2007;10:35–45. doi: 10.1007/s10456-006-9061-x. [DOI] [PubMed] [Google Scholar]
- 40.Tigges U, Hyer E, Scharf J, Stallcup W. FGF2-dependent neovascularization of subcutaneous Matrigel plugs is initiated by bone marrow-derived pericytes and macrophages. Development. 2008;135:523–532. doi: 10.1242/dev.002071. [DOI] [PubMed] [Google Scholar]
- 41.Levine J, Stincone F, Lee Y. Development and differentiation of glial progenitor cells in the rat cerebellum. Glia. 1993;7:307–321. doi: 10.1002/glia.440070406. [DOI] [PubMed] [Google Scholar]
- 42.Nishiyama A, Lin X, Giese N, Heldin C, Stallcup W. Co-localization of NG2 proteoglycan and PDGF alpha receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 43.Nishiyama A, Lin X, Giese N, Heldin C, Stallcup W. Interaction between NG2 proteoglycan and PDGF alpha receptor on O2A cells is required for optimal response to PDGF. J Neurosci Res. 1996;43:315–330. doi: 10.1002/(SICI)1097-4547(19960201)43:3<315::AID-JNR6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 44.Trapp B, Nishiyama A, Cheng D, Macklin W. Differentiation and death of premyelinating oligodendrocytes in developing rodent brain. J Cell Biol. 1997;137:459–468. doi: 10.1083/jcb.137.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reynolds R, Hardy R. Oligodendrocyte progenitors labeled with the O4 antibody persist in the adult rat cerebral cortex. J Neurosci Res. 1997;47:455–470. doi: 10.1002/(sici)1097-4547(19970301)47:5<455::aid-jnr1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 46.Keirstead H, Levine J, Blakemore W. Response of oligodendrocyte progenitor cell population (defined by NG2 labeling) to demyelination of the adult spinal cord. Glia. 1998;22:161–170. [PubMed] [Google Scholar]
- 47.Luskin M, Parnevelas J, Barfield J. Neurons, astrocytes and oligodendrocytes of the rat cerebral cortex originate from separate progenitor cells: An ultrastructural analysis of clonally related cells. J Neurosci. 1993;13:1730–1750. doi: 10.1523/JNEUROSCI.13-04-01730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levison S, Young G, Goldman J. Cycling cells in the adult rat neocortex preferentially generate oligodendroglia. J Neurosci Res. 1999;57:435–446. [PubMed] [Google Scholar]
- 49.Zhu X, Bergles D, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1017/S1740925X09000015. [DOI] [PubMed] [Google Scholar]
- 50.Raff M. Glial cell diversification in the optic nerve. Science. 1989;243:1450–1455. doi: 10.1126/science.2648568. [DOI] [PubMed] [Google Scholar]
- 51.Richardson W, Raff M, Noble M. The oligodendrocyte/type II astrocyte lineage. Semin Neurosci. 1990;2:445–454. [Google Scholar]
- 52.Stallcup W, Beasley L. Bipotential glial precursor cells of the optic nerve express the NG2 proteoglycan. J Neurosci. 1987;2001:2737–2744. doi: 10.1523/JNEUROSCI.07-09-02737.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Horner P, Power A, Kempermann G, Kuhn H, Palmer T, Winker J, Thal L, Gage F. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gensert J, Goldman J. In vivo characterization of endogenous proliferating cells in adult rat subcortical white matter. Glia. 1996;17:39–51. doi: 10.1002/(SICI)1098-1136(199605)17:1<39::AID-GLIA4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 55.Gensert J, Goldman J. Heterogeneity of cycling glial progenitors in the adult mammalian cortex and white matter. J Neurobiol. 2001;48:75–86. [PubMed] [Google Scholar]
- 56.Nishiyama A, Watanabe M, Yang Z, Bu J. Identity, distribution and development of polydendrocytes: NG2-expressing glial cells. J Neurocytol. 2002;31:437–455. doi: 10.1023/a:1025783412651. [DOI] [PubMed] [Google Scholar]
- 57.Peters A. A fourth type of neuroglial cell in the adult central nervous system. J Neurocytol. 2004;33:345–357. doi: 10.1023/B:NEUR.0000044195.64009.27. [DOI] [PubMed] [Google Scholar]
- 58.Gensert J, Goldman J. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- 59.Keirstead H, Blakemore W. Identification of postmitotic oligodendrocytes incapable of remyelination within the demyelinated adult spinal cord. Neuropathol Exp Neurol. 1997;56:1191–1201. doi: 10.1097/00005072-199711000-00003. [DOI] [PubMed] [Google Scholar]
- 60.Levine J, Reynolds R. Activation and proliferation of endogenous oligodendrocyte progenitor cells during ethidium bromide-induced demyelination. Exp Neurol. 1999;160:333–347. doi: 10.1006/exnr.1999.7224. [DOI] [PubMed] [Google Scholar]
- 61.McTigue D, Wei P, Stokes B. Proliferation of NG2 positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci. 2001;21:3392–3400. doi: 10.1523/JNEUROSCI.21-10-03392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ong W, Levine J. A light and electron microscopic study of NG2 chondroitin sulfate proteoglycan positive oligodendrocyte precursor cells in the normal and kainite-lesioned rat hippocampus. Neurosci. 1999;92:83–95. doi: 10.1016/s0306-4522(98)00751-9. [DOI] [PubMed] [Google Scholar]
- 63.Butt A, Duncan A, Hornby N, Kirvell S, Hunter A, Levine J, Berry M. Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia. 1999;26:84–91. [PubMed] [Google Scholar]
- 64.Butt A, Kiff J, Hubbard P, Berry M. Synantocytes: new functions for novel NG2 expressing glia. J Neurocytol. 2002;31:551–565. doi: 10.1023/a:1025751900356. [DOI] [PubMed] [Google Scholar]
- 65.Bergles D, Roberts J, Somogyi P, Jahr C. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- 66.Lin S, Bergles D. Physiological characteristics of NG2-expressing glial cells. J Neurocytol. 2002;31:537–549. doi: 10.1023/a:1025799816285. [DOI] [PubMed] [Google Scholar]
- 67.Paukert M, Bergles D. Synaptic communication between neurons and NG2+ cells. Curr Opin Neurobiol. 2006;16:515–521. doi: 10.1016/j.conb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 68.Bachoo R, Maher E, Ligon K, Sharpless N, Chan S, You M, Tang Y, DeFrances J, Stover E, Weissleder R, Rowitch D, Louis D, DePinho R. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269–277. doi: 10.1016/s1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 69.Singh S, Hawkins C, Clarke I, Squire J, Bayani J, Hide T, Henkelman R, Cusimano M, Dirks P. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 70.Sanai N, Alvarez-Buylla A, Berger M. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353:811–8269. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 71.Dai C, Celestino J, Okada Y, Louis D, Fuller G, Holland E. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2006;15:1913–1925. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schrappe M, Klier F, Spiro R, Waltz T, Reisfeld R, Gladson C. Correlation of chondroitin sulfate proteoglycan expression on proliferating brain capillary endothelial cells with the malignant phenotype of asdtroglial cells. Cancer Res. 1991;51:4986–4993. [PubMed] [Google Scholar]
- 73.Shoshan Y, Nishiyama A, Chang A, Mork S, Barnett G, Cowell J, Trapp B, Staugaitis S. Expression of oligodendrocyte progenitor cell antigens by gliomas: implications for the histogenesis of brain tumors. Proc Natl Acad Sci USA. 1999;96:10361–10366. doi: 10.1073/pnas.96.18.10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chekenya M, Rooprai H, Davies D, Levine J, Butt A, Pilkington G. The NG2 chondroitin sulfate proteoglycan: role in malignant progression of human brain tumors. Int J Dev Neurosci. 1999;17:421–435. doi: 10.1016/s0736-5748(99)00019-2. [DOI] [PubMed] [Google Scholar]
- 75.Dawson M, Polito A, Levine J, Reynolds R. NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors? J Neurosci Res. 2000;61:471–479. doi: 10.1002/1097-4547(20000901)61:5<471::AID-JNR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 76.Mason J, Goldman J. A2B5+ and O4+ cycling progenitors in the adult forebrain white matter respond differentially to PDGF-AA, FGF-2 and IGF-1. Mol Cell Neurosci. 2002;20:30–42. doi: 10.1006/mcne.2002.1114. [DOI] [PubMed] [Google Scholar]
- 77.Roy N, Wang S, Harrison-Restelli C, Benraiss A, Fraser R, Gravel M, Braun P, Goldman S. Identification, isolation and promoter-defined separation of mitotic oligodendrocyte progenitor cells from the adult human subcortical white matter. J Neurosci. 1999;19:9986–9995. doi: 10.1523/JNEUROSCI.19-22-09986.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uhrbom L, Hesselager G, Ostman A, Nister M, Westermark B. Dependence of autocrine growth factor stimulation in PDFG-B-induced mouse brain tumor cells. Int J Cancer. 2000;85:398–406. doi: 10.1002/(sici)1097-0215(20000201)85:3<398::aid-ijc17>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 79.Hesselager G, Uhrbom L, Westermark B, Nister M. Complementary effects of PDGF autocrine stimulation and p53 or Ink4a-Arf deletion in a mouse glioma model. Cancer Res. 2003;63:4305–4309. [PubMed] [Google Scholar]
- 80.Shih A, Holland E. PDGF and glial tumorigenesis. Cancer Lett. 2006;232:139–147. doi: 10.1016/j.canlet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 81.Chekenya M, Hjelstuen M, Enger P, Thorsen F, Jacob A, Probst B, Haraldseth O, Pilkington G, Butt A, Levine J, Bjerkvig R. NG2 proteogycan promotes angiogenesis-dependent tumor growth in CNS by sequestering angiostatin. FASEB J. 2002;16:586–588. doi: 10.1096/fj.01-0632fje. [DOI] [PubMed] [Google Scholar]
- 82.Chekenya M, Enger P, Thorsen F, Tysnes B, Al-Sarraj S, Read T, Furmanek T, Mahesparan R, Levine J, Butt A, Pilkington G, Bjerkvig R. The glial precursor proteoglycan NG2 is expressed on tumor neovasculature by vascular pericytes in human malignant brain tumours. Neuropathol Appl Neurobiol. 2002;28:367–380. doi: 10.1046/j.1365-2990.2002.00412.x. [DOI] [PubMed] [Google Scholar]
- 83.Wiranowska M, Ladd S, Smith S, Gottschall P. CD44 adhesion molecule and neuroglial proteoglycan NG2 as invasive markers of glioma. Brain Cell Biol. 2006;35:159–172. doi: 10.1007/s11068-007-9009-0. [DOI] [PubMed] [Google Scholar]
- 84.Chekenya M, Krakstad C, Svendsen A, Netland I, Staalsen V, Tysnes B, Selheim F, Wang J, Sakariassen P, Sandal T, Lonning P, Flatmark T, Enger P, Bjerkvig R, Sioud M, Stallcup W. The progenitor cell marker NG2/MPG promotes chemoresistance by activation of integrin-dependent PI3K/Akt signaling. Oncogene. 2008;27:5182–5194. doi: 10.1038/onc.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Assanah M, Lochhead R, Ogden A, Bruce J, Goldman J, Canoll P. Glial progenitors in adult white matter are driven to form malignant gliomas by PDGF expressing retroviruses. J Neurosci. 2006;26:6781–6790. doi: 10.1523/JNEUROSCI.0514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Westermark B, Heldin C, Nister M. Platelet-derived growth factor in human glioma. Glia. 1995;15:257–263. doi: 10.1002/glia.440150307. [DOI] [PubMed] [Google Scholar]
- 87.Van der Valk P, Lindeman J, Kamphorst W. Growth factor profiles of human gliomas. Do non-tumour cells contribute to tumour growth in glioma? Ann Oncol. 1997;8:1023–1029. doi: 10.1023/a:1008265905505. [DOI] [PubMed] [Google Scholar]
- 88.Schlingemann R, Rietveld F, Kwaspen F, van de Kerkhof P, de Waal R, Ruiter D. Differential expression of markers for endothelial cells, pericytes and basal lamina in the microvasculature of tumors and granulation tissue. Am J Pathol. 1991;138:1335–1347. [PMC free article] [PubMed] [Google Scholar]
- 89.Song S, Ewald A, Stallcup W, Werb Z, Bergers G. PDGFRβ+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nature Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morikawa S, Baluk P, Kaidoh T, et al. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burger P, Kleihues P. Cytologic composition of the untreated glioblastoma with implications for evaluation of needle biopsies. Cancer. 1989;63:2014–2023. doi: 10.1002/1097-0142(19890515)63:10<2014::aid-cncr2820631025>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 92.Wesseling P, Schlingemann R, Rietveld F, Link M, Burger P, Ruiter D. Early and extensive contribution of pericytes/vascular smooth muscle cells to microvascular proliferation in glioblastoma multiforme: an immuno-light and immuno-electron microscopic study. J Neuropathol Exp Neurol. 1995;54:304–310. doi: 10.1097/00005072-199505000-00003. [DOI] [PubMed] [Google Scholar]
- 93.Brekke C, Lundervold A, Enger P, Brekken C, Stalsett E, Pedersen T, Haraldseth O, Kruger P, Bjerkvig R, Chekenya M. NG2 expression regulates vascular morphology and function in human brain tumors. NeuroImage. 2006;29:965–976. doi: 10.1016/j.neuroimage.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 94.Burg M, Grako K, Stallcup W. Expression of the NG2 proteoglycan enhances the growth and metastatic properties of melanoma cells. J Cell Physiol. 1998;177:299–312. doi: 10.1002/(SICI)1097-4652(199811)177:2<299::AID-JCP12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 95.Real F, Houghton A, Albino A, Cordon-Cardo C, Melamid M, Oettgen H, Old L. Surface antigens of melanomas and melanocytes defined by mouse monoclonal antibodies: specificity analysis and comparison of antigen expression in cultured cells and tissues. Cancer Res. 1985;45:4401–4411. [PubMed] [Google Scholar]
- 96.Leger O, Johnson-Leger C, Jackson E, Coles B, Dean C. The chondroitin sulfate proteoglycan NG2 is a tumour-specific antigen on the chemically induced rat chondrosarcoma HSN. Int J Cancer. 1994;58:700–705. doi: 10.1002/ijc.2910580514. [DOI] [PubMed] [Google Scholar]
- 97.Smith F, Rauch C, Williams D, March C, Arthur D, et al. The human homolog of rat NG2, a chondroitin sulfate proteoglycan, is not expressed on the cell surface of normal hematopoietic cells but is expressed by acute myeloid leukemia blasts from poor prognosis patients with abnormalities of human chromosome band 11q23. Blood. 1996;87:1123–1133. [PubMed] [Google Scholar]
- 98.Couchman J. Syndecans: proteoglycan regulators of cell-surface microdomains? Nature Rev Mol Cell Biol. 2003;4:926–938. doi: 10.1038/nrm1257. [DOI] [PubMed] [Google Scholar]
- 99.Fang X, Burg M, Barritt D, Dahlin-Huppe K, Nishiyama A, Stallcup W. Cytoskeletal reorganization induced by engagement of the NG2 proteoglycan leads to cell spreading and migration. Mol Biol Cell. 1999;10:3373–3387. doi: 10.1091/mbc.10.10.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Majumdar M, Vuori K, Stallcup W. Engagement of NG2 proteoglycan triggers cell spreading via rac and p130cas. Cell Signalling. 2003;15:79–84. doi: 10.1016/s0898-6568(02)00045-1. [DOI] [PubMed] [Google Scholar]
- 101.Tillet E, Gential B, Garrone R, Stallcup W. The NG2 proteoglycan mediates b1 integrin-independent cell adhesion and spreading on collagen VI. J Cell Biochem. 2002;86:726–736. doi: 10.1002/jcb.10268. [DOI] [PubMed] [Google Scholar]
- 102.Bumol T, Walker L, Reisfeld R. Biosynthetic studies of proteoglycans in human melanoma cells with a monoclonal antibody to a core glycoprotein of chondroitin sulfate proteoglycams. J Biol Chem. 1984;259:12733–12741. [PubMed] [Google Scholar]
- 103.Harper J, Reisfeld R. Cell-associated proteoglycans in human malignant melanoma. In: Wight T, Mecham R, editors. Biology of Proteoglycans. San Diego CA: Academic Press Inc.; 1987. pp. 345–366. [Google Scholar]
- 104.Lin X, Dahlin-Huppe K, Stallcup W. Interaction of the NG2 proteoglycan with the actin cytoskeleton. J Cell Biochem. 1996;63:463–477. doi: 10.1002/(sici)1097-4644(19961215)63:4<463::aid-jcb8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 105.Lin X, Grako K, Burg M, Stallcup W. NG2 proteoglycan and the actin-binding protein fascin define separate populations of actin-containing filopodia and lamellipodia during cell spreading and migration. Mol Biol Cell. 1996;7:1977–1993. doi: 10.1091/mbc.7.12.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stallcup W, Dahlin K, Healy P. Interaction of the NG2 chondroitin sulfate proteoglycan with type VI collagen. J Cell Biol. 1990;111:3177–3188. doi: 10.1083/jcb.111.6.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nishiyama A, Stallcup W. Expression of NG2 proteoglycan causes retention of type VI collagen on the cell surface type. Mol Biol Cell. 1993;4:1097–1108. doi: 10.1091/mbc.4.11.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Burg M, Tillet E, Timpl R, Stallcup W. Binding of the NG2 proteoglycan to type VI collagen and other extracellular matrix ligands. J Biol Chem. 1996;271:26110–26116. doi: 10.1074/jbc.271.42.26110. [DOI] [PubMed] [Google Scholar]
- 109.Farin A, Suzuki S, Weiker M, Goldman J, Bruce J, Canoll P. Transplanted glioma cells migrate and proliferate on host brain vasculature: a dynamic analysis. Glia. 2006;53:799–808. doi: 10.1002/glia.20334. [DOI] [PubMed] [Google Scholar]
- 110.Hagg T, Portera-Cailliau C, Jucker M, Engvall E. Laminins of the adult mammalian CNS: laminin alpha-2 (merosin M-) chain immunoreactivity is associated with neuronal processes. Brain Res. 1997;764:17–27. doi: 10.1016/s0006-8993(97)00419-8. [DOI] [PubMed] [Google Scholar]
- 111.Buttery P, Ffrench-Constant C. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol Cell Neurosci. 1999;14:199–212. doi: 10.1006/mcne.1999.0781. [DOI] [PubMed] [Google Scholar]
- 112.Fukushi J, Makagiansar I, Stallcup W. NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and α3β1 integrin. Mol Biol Cell. 2004;15:3580–3590. doi: 10.1091/mbc.E04-03-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Iida J, Meijne J, Spiro R, Roos E, Furcht L, McCarthy J. Spreading and focal contact formation of human melanoma cells in response to the stimulation of melanoma-associated proteoglycan (NG2) and alpha 4, beta 1 integrin. Cancer Res. 1995;55:2177–2185. [PubMed] [Google Scholar]
- 114.Eisenmann K, McCarthy J, Simpson M, Keely P, Guan J, Tachibana K, Lim L, Manser E, Furscht L, Iida J. Melanoma chondroitin sulfate proteoglycan regulates cell spreading through cdc42, Ack-1 and p130cas. Nature Cell Biol. 1999;1:507–513. doi: 10.1038/70302. [DOI] [PubMed] [Google Scholar]
- 115.Yang J, Price M, Neudauer C, Wilson C, Ferrone S, Xia H, Iida J, Simpson M, McCarthy J. Melanoma chondroitin sulfate proteoglycan enhances FAK and ERK activation by distinct mechanisms. J Cell Biol. 2004;165:881–891. doi: 10.1083/jcb.200403174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Luque A, Gomez M, Puzon W, Takada Y, Sanchez-Madrid F, Cabanas C. Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region of the common beta 1 chain. J Biol Chem. 1996;271:11067–11075. doi: 10.1074/jbc.271.19.11067. [DOI] [PubMed] [Google Scholar]
- 117.Goretzki L, Burg M, Grako K, Stallcup W. High affinity binding of bFGF and PDGF-AA to the core protein of the NG2 proteoglycan. J Biol Chem. 1999;274:16831–16837. doi: 10.1074/jbc.274.24.16831. [DOI] [PubMed] [Google Scholar]
- 118.Rapraeger A. In the clutches of proteoglycans: how does heparan sulfate regulate FGF binding? Curr Biol. 1995;2:645–649. doi: 10.1016/1074-5521(95)90025-x. [DOI] [PubMed] [Google Scholar]
- 119.Stallcup W. The NG2 proteoglycan: past insights and future prospects. J Neurocytol. 2002;31:423–435. doi: 10.1023/a:1025731428581. [DOI] [PubMed] [Google Scholar]
- 120.Grako K, Ochiya T, Barritt D, Nishiyama A, Stallcup W. PDGF alpha receptor is unresponsive to PDGF-AA in aortic smooth muscle cells from the NG2 knockout mouse. J Cell Sci. 1999;112:905–915. doi: 10.1242/jcs.112.6.905. [DOI] [PubMed] [Google Scholar]
- 121.Ozerdem U, Stallcup W. Pathological angiogenesis is reduced by targeting pericytes via the NG2 proteoglycan. Angiogenesis. 2004;7:269–276. doi: 10.1007/s10456-004-4182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Downward J. PI3-kinase, Akt and cell survival. Semin Cell Dev Biol. 2004;15:177–182. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 123.Joy A, Beaudry C, Tran N, Ponce F, Holz D, Demuth T, Berens M. Migrating glioma cells activate the PI3K pathway and display decreased susceptibility to apoptosis. J Cell Sci. 2003;116:4409–4417. doi: 10.1242/jcs.00712. [DOI] [PubMed] [Google Scholar]
- 124.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 125.Nehls V, Denzer K, Drenkhahn D. Pericyte involvement in capillary sproutingduring angiogenesis in situ. Cell Tiss Res. 1992;270:469–474. doi: 10.1007/BF00645048. [DOI] [PubMed] [Google Scholar]
- 126.Redmer D, Doraiswamy V, Bortnem B, Fisher K, Jablonka-Sharrif A, Grazul-Bilska A, Reynolds L. Evidence for a role of capillary pericytes in vascular growth of the developing ovine corpus luteum. Biol Reprod. 2001;65:879–889. doi: 10.1095/biolreprod65.3.879. [DOI] [PubMed] [Google Scholar]
- 127.Lindahl P, Johansson B, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 128.Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Enge M, Bjarnegard M, Gerhardt H, Gustafsson E, Kalen M, Asker N, Hammes H, Shani M, Fassler R, Betsholtz C. Endothelium specific platelet derived growth factor B ablation mimics diabetic retinopathy. EMBO J. 2002;21:4307–4316. doi: 10.1093/emboj/cdf418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Goretzki L, Lombardo C, Stallcup W. Binding of the NG2 proteoglycan to kringle domains modulates the functional properties of angiostain and plasmin(ogen) J Biol Chem. 2000;275:28625–29633. doi: 10.1074/jbc.M002290200. [DOI] [PubMed] [Google Scholar]
- 131.Webster M, Hutchinson J, Rauh M, Muthaswamy S, Anton M, Tortorice C, Cardiff R, Graham F, Hassel J, Muller W. Requirement for both Shc and PI3kinase signaling pathways in polyome middle T-mediated mammary tumorigenesis. Mol Cell Biol. 1998;18:2344–2359. doi: 10.1128/mcb.18.4.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Maglione J, Moghanaki D, Young L, Manner C, Ellies L, Joseph S, Nicholson N, Cardiff R, MacLeod C. Transgenic polyoma middle T mice model pre-malignant mammary disease. Cancer Res. 2001;61:8298–8305. [PubMed] [Google Scholar]
- 133.Seyfried T, el-Abbadi M, Roy M. Gangioside distribution in murine neural tumors. Mol Chem Neuropathol. 1992;17:147–167. doi: 10.1007/BF03159989. [DOI] [PubMed] [Google Scholar]
- 134.Seyfried N, Huysentruyt L, Atwood J, Xia Q, Seyfried T, Orlando R. Upregulation of NG2 proteoglycan and interferon-induced transmembrane proteins 1 and 3 in mouse astrocytoma: a membrane proteomics approach. Cancer Lett. 2008;263:243–252. doi: 10.1016/j.canlet.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]