Abstract
Desmosomes are intercellular junctions responsible for strong cell-cell adhesion in epithelia and cardiac muscle. Numerous studies have shown that the other major type of epithelial cell adhesion, the adherens junction, is destabilized by src-induced tyrosine phosphorylation of two of its principal components, E-cadherin and β-catenin. Here we show that treatment of epithelial cells with the potent tyrosine phosphatase inhibitor sodium pervanadate causes tyrosine phosphorylation of the major desmosomal components desmoglein 2 and plakoglobin in both the non-ionic detergent soluble and insoluble cell fractions and, surprisingly, stabilizes desmosomal adhesion, inducing the hyper-adhesive form normally found in tissues and confluent cell sheets. Taken together with the few other studies on desmosomes these results suggest that the effects of tyrosine phosphorylation on desmosomal adhesion are complex.
Key words: desmosome, cell-cell adhesion, intercellular junction, tyrosine phosphorylation, pervanadate, desmoglein, plakoglobin
Introduction
Desmosomes are intercellular junctions responsible for strong cell-cell adhesion especially in epithelia and cardiac muscle.1–3 Their adhesiveness is regulated by the serine/threonine kinase protein kinase C.2,4,5 Thus in tissues and confluent cultured cell sheets desmosomes are hyper-adhesive, meaning that they are strongly adhesive and resistant to disruption by chelating agents even though their adhesion is mediated by the calcium-dependent desmosomal cadherins, desmocollin and desmoglein. When epithelia are wounded their desmosomes revert to a more weakly adhesive, calcium-dependent form and this change is mediated by activation of protein kinase C.
The other major epithelial adhesive junction, the adherens junction, remains permanently calcium-dependent as far as is known. Its adhesiveness has been shown to be disrupted by tyrosine phosphorylation of two of its major components, E-cadherin and β-catenin6–11 Such a mechanism, mediated by the tyrosine kinase c-src, is believed to be important in promoting certain types of human cancer, for example colorectal carcinoma.12–15 One aspect of this promotion may involve disruption of cell adhesion leading to increased cell motility, invasion and metastasis. As well as negative regulation of adhesion by tyrosine kinases, low molecular weight tyrosine phosphatase has been shown to associate with E-cadherin and to potentiate cell-cell adhesion.16
Loss of cell adhesion in epithelia must involve interruption of desmosomal adhesion as well as that of adherens junctions so it is important to know whether desmosomal adhesion is also affected by tyrosine phosphorylation but there have been few studies in this area. Treatment of cells of the human epidermoid carcinoma cell line A431 with EGF induces tyrosine phosphorylation of the armadillo protein plakoglobin (Pg), a component of desmosomes and adherens junctions, and the desmosomal cadherin desmoglein 2 (Dsg2), together with a modest decrease in cytoskeletal association of Pg and increase in the cytoplasm.17,18 Furthermore tyrosine-phosphorylated Pg does not associate with the desmosomal plaque protein desmoplakin (DP). These effects are essentially blocked by treatment with EGFR inhibitor.19 The interaction of Pg with adherens junction components is modified by tyrosine phosphorylation in a manner that varies according to which site is phosphorylated. Thus src phosphorylates Tyr 643 decreasing Pg binding to E-cadherin and α-catenin whereas Fer phosphorylates Tyr 549 increasing Pg binding to α-catenin.20
In order to investigate the effect of tyrosine phosphorylation on desmosomal adhesion further we have treated Madin-Darby Canine Kidney cells (MDCK) with the potent tyrosine phosphatase inhibitor sodium pervanadate. This and related treatments have previously been shown to cause tyrosine phosphorylation of adherens junction components and loss of adhesion.8,21 Surprisingly, pervanadate treatment both caused phosphorylation of desmosomal components and stabilized desmosomal adhesion so that they resembled the hyper-adhesive desmosomes found in confluent cell sheets and tissues, in contrast to the destabilizing effects found previously on adherens junctions.
Results
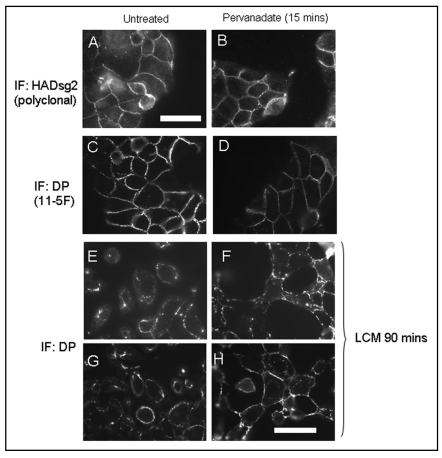
Working with subconfluent MDCK cells, which always have calcium-dependent, non-hyper-adhesive desmosomes, we first showed that treatment with pervanadate for 15 minutes had no apparent effect on cell shape or on the distribution of desmosomal proteins (Fig. 1A–D). In order to test whether the adhesiveness and stability of desmosomes was altered by pervanadate treatment cells were exposed to low calcium medium (LCM) for 90 minutes either untreated or after a 15 minute exposure to pervanadate. It was found that untreated control cells showed internalization of desmosomal components as previously described (Mattey and Garrod, 1986; Wallis et al., 2000; Kimura et al., 2007). By contrast, pervanadate-treated cells showed retention of desmosomes at the cell surface at points of cell-cell contact even though there was considerable loss of intercellular adhesion elsewhere on the cell surface (Fig. 1E–H). Their appearance was typical of cells with hyper-adhesive desmosomes as described in the above references. Quantification of the effect showed that 40–60% of cells possessed hyper-adhesive, calcium-independent desmosomes after pervanadate treatment in different experiments, whereas in untreated controls the figure was <1%. Thus pervanadate treatment appears to stabilize desmosomal adhesion.
Figure 1.
(A–D). Pervanadate treatment does not affect distribution of desmosomal proteins. MDCK cells expressing mDsg2HA (see Fig. 2) were plated at a density of 1.25 × 104 cells/cm2. After 48 hours the subconfluent cultures were either untreated (A and C) or treated for 15 minutes with pervanadate prior to fixation and processing for indirect immunofluorescence (B and D). Exogenous Dsg2 was stained using a polyclonal anti-HA antibody and DP monoclonal antibody 11–5F. Pervanadate treatment for this duration caused no apparent alteration in the junctional distribution of either ectopic HAmDsg2 or endogenous DP. (E–H) Stabilization of desmosomes by pervanadate. Subconfluent cultures were either untreated or pretreated with pervanadate for 15 minutes and then switched to low-calcium medium (LCM) for 90 minutes. Switching untreated cells to LCM caused them to lose the junctional distribution of DP as desmosomes were internalized and cells detached from one another (E and G). Pervanadate-treated cells switched to LCM showed intense DP staining at areas of mutual adhesion even though there was considerable retraction at other parts of the cell surface (F and H). Bars = 25 µm. That in (A) is for (A–D) and that in (H) for (E–H).
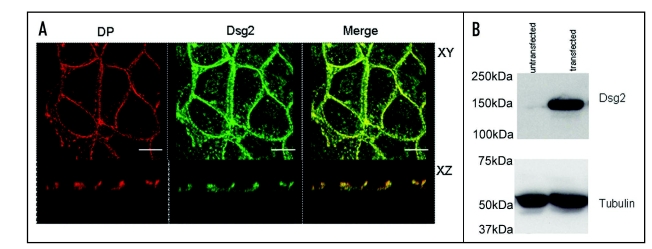
In order make biochemical observations involving pull-down assays on pervanadate-treated cells we used a stable cell line that had been transfected with the HA-tagged desmosomal cadherin mouse desmoglein 2 (mDsg2). These cells are referred to as MDCK-HAmDsg2. Figure 2 shows that the exogenous mDsg2 colocalized with the endogenous desmosomal plaque protein DP by immunofluorescence and confocal microscopy indicating that it was located in desmosomes (Fig. 2A). Immunoblotting showed that the exogenous protein was strongly expressed (Fig. 2B).
Figure 2.
Exogenous Dsg2 colocalizes with DP and is strongly expressed. (A) Confocal images of MDCK cells with stable expression of mDsg2HA stained for DP with monoclonal antibody 11–5F (left) and polyclonal anti-HA antibody (centre). The merged image (right) shows that Dsg2 is colocalized with DP in both the XY and XZ planes. (B) Immunoblots of equally loaded gel (tubulin control) showing strong expression of mDsg2HA detected with polyclonal anti-HA antibody. Bars = 5 µm.
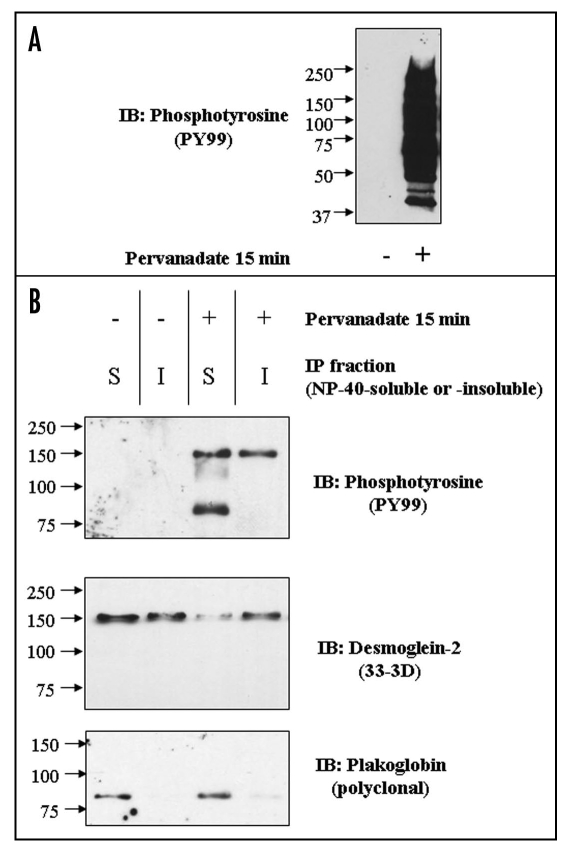
Immunoblotting of whole extracts of control cells and cells treated with pervanadate for 15 minutes with antibody to phosphotyrosine showed that pervanadate induced massive phosphorylation of cellular proteins (Fig. 3A). Immunoprecipitation (IP) from NP-40-soluble and insoluble extracts of control cells and pervanadate-treated cells with anti-HA antibody followed by immunoblotting with anti-phosphotyrosine antibody showed an absence of phosphorylated proteins in the control cells (Fig. 3B). By contrast in the soluble fraction from pervanadate-treated cells two tyrosine-phosphorylated polypeptides or relative mobilities approximating to 150 kDa and 80 kDa were detected, of which the larger was also present in the IP from the insoluble fraction. The 150 kDa polypeptide was detected by a monoclonal antibody to Dsg2 and was also present in both fractions obtained from control cells. The 80 kDa protein was detected by an antibody to Pg in the soluble fractions of both control and pervanadate-treated cells. These results show that Dsg2 and Pg are present as a complex in the soluble fraction of both control and pervanadate-treated cells, that both proteins become tyrosine-phosphorylated in the presence of pervanadate, and that phosphorylation does not disrupt their complex.
Figure 3.
(A) Pervanadate induces extensive tyrosine phosphorylation of cellular proteins. Equally loaded immunoblots of whole cell extracts untreated (left) and pervanadate-treated (right) MDCK cells with anti-phosphotyrosine antibody PY99. (B) Pervanadate causes tyrosine phosphorylation of Dsg2 and Pg, which co-precipitate from the NP-40-soluble fraction. Cultures were either untreated or treated with pervanadate for 15 minutes prior to detergent fractionation (S = soluble; I = insoluble) and subsequent immunoprecipitation of ectopic mDsg2HA. Samples were fractionated by SDS-PAGE and immunoblotted with an anti-phosphotyrosine antibody. The membrane was then sequentially re-probed for mDsg2HA and Pg.
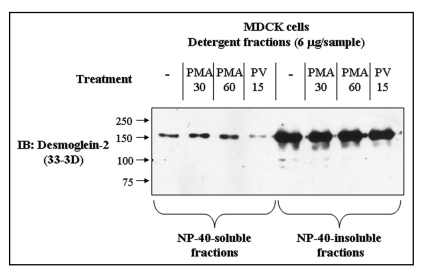
A consistent observation was that the amount of Dsg2 in immunoprecipitates from the soluble fraction of pervanadate-treated cells was less than that obtained from control cells (Fig. 3B for example). Immunoblots of soluble and insoluble extracts confirmed this observation (Fig. 4). Treatment of the cells with the phorbol ester PMA, which activate PKC, a known regulator of desmosomal adhesiveness, produced no change in the amount of Dsg2 in the soluble fraction. The amount of Dsg2 in the insoluble fraction remained approximately constant under all conditions tested. Since the amount of Pg in the IP from the soluble fraction of both control and pervanadate-treated cells remained approximately the same (Fig. 3B), it may be that rather than disrupting the Dsg2-Pg complex, pervanadate treatment actually slightly enhances the interaction.
Figure 4.
Pervanadate treatment reduces the amount of Dsg2 in the soluble fraction. Confluent cultures of untransfected MDCK cells were untreated, treated with pervanadate for 15 mins (PV15) or treated with 50 nM phorbol myristate acetate for 30 (PMA 30) or 60 minutes (PMA 60). Following the treatments the cells were fractioned into NP-40-soluble and -insoluble fractions and equal quantities of protein subjected to SDS-PAGE and immunoblotting for Dsg2. PMA treatment did not alter the level of Dsg2 in either of the detergent fractions whereas pervanadate treatment reduced the amount of Dsg2 in the soluble fraction.
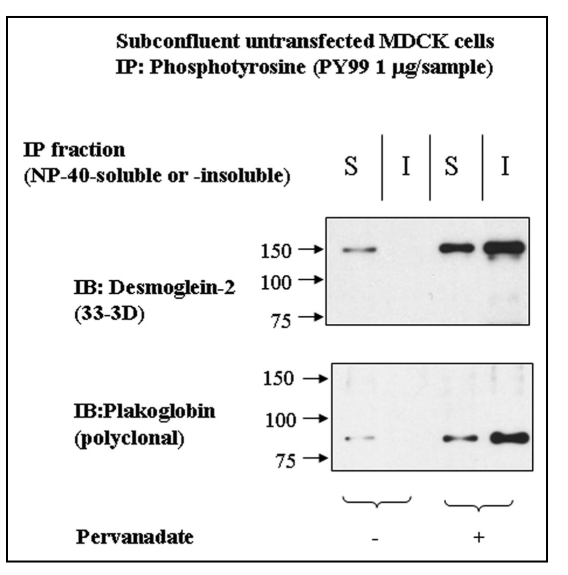
Because all experiments so far concentrated on the phosphorylation of exogenous Dsg2 and its associated Pg, we determined whether the endogenous protein also became tyrosine-phosphorylated by pervanadate treatment. The soluble and insoluble fractions of untransfected control and pervanadate-treated MDCK cells were immunoprecipitated with anti-phosphotyrosine antibody and the products immunoblotted for Dsg2 and Pg. Both fractions from the pervanadate-treated cells showed the presence of tyrosine-phosphorylated Dsg2 and Pg (Fig. 5). Surprisingly the soluble fraction of control cells showed small amounts of both proteins (Fig. 5), indicating that a proportion of Dsg2 and Pg in the NP40-soluble fraction was tyrosine-phosphorylated in subconfluent MDCK cells under control conditions.
Figure 5.
Dsg2 and Pg are slightly tyrosine-phosphorylated in the insoluble fraction of control cells. Untransfected MDCK cells were either untreated or treated with pervanadate for 15 minutes prior to detergent fractionation and subsequent immunoprecipitation using the monoclonal anti-phosphotyrosine antibody PY99. Samples were fractionated with equal loading by SDS-PAGE and immunoblotted for endogenous Dsg2 and Pg. As expected Dsg2 and Pg in the pervanadate-treated samples were tyrosine-phosphorylated. There was also tyrosine-phosphorylated Dsg2 and Pg in the soluble fraction of untreated cells yet apparently none in the insoluble fraction. As this population of protein was undetectable within the ectopic mDsg2HA IPs it must be assumed that it represents a small proportion of the cellular pool of these proteins.
Discussion
In view of a substantial amount of data showing that tyrosine phosphorylation destabilizes adherens junctions (see Introduction) it was surprising to find that pervanadate treatment stabilized desmosomes, increasing their adhesiveness and resistance to calcium chelation. This was especially so since previous studies involving activation/inhibition of EGF signalling, though not producing dramatic effects, indicated that tyrosine phosphorylation tended to destabilize desmosomal adhesion.17–19 On the other hand, the work of Miravet et al. (2003) showed that phosphorylation of Pg at different sites can stabilize or destabilize its interaction with adherens junction components.20 It is also of interest that the non-receptor tyrosine kinase Fyn, which phosphorylates β-catenin and Pg, has been associated with the promotion of keratinocyte adhesion.25 Taken together with previous observations our results suggest that tyrosine phosphorylation can have opposing effects on desmosomal adhesion.
Our biochemical analysis shows that pervanadate induced tyrosine phosphorylation of Dsg2 and Pg in both the NP40-soluble and -insoluble fractions. This contrasts with the work of Gaudry et al.17 who found Pg tyrosine phosphorylated through the EGF receptor exclusively in the non-ionic detergent-soluble fraction. The proteins in the insoluble fraction are presumably largely or entirely located in desmosomes, a suggestion consistent with our observation that exogenous HA-tagged Dsg2 was co-localized with the desmosomal plaque marker DP by confocal microscopy. Indeed, Kimura et al.2 showed that the molecular composition of both calcium dependent and hyper-adhesive desmosomes to be identical. In particular, both contained Pg and desmoplakin. This again appears to contrast with the work of Gaudry et al.17 who found no association between Pg tyrosine phosphorylated through the EGF receptor and desmoplakin. A further contrast between our results and those obtained with the EGF signalling system is that inhibition of the EGF receptor caused initiation of desmosome assembly even in low calcium medium,19 whereas pervanadate-induced phosphorylation rendered desmosomes resistant to calcium chelation.
Tyrosine phosphorylation of desmosomes has previously been demonstrated by immunogold labelling and electron microscopy.26 This is important because it is clearly phosphorylation events that occur in desmosomes that directly regulate their adhesiveness. We have shown previously that PKCα, activation of which converts desmosomes from the hyper-adhesive form to calcium dependence, is a component of the cytoplasmic plaques of calcium-dependent desmosomes.5 We may speculate that a tyrosine kinase and phosphatase are also located in the desmosomal plaque under some circumstances. Furthermore, it appears that both serine/threonine and tyrosine phosphorylation can be involved in regulating desmosomal adhesion, but the former destabilizes and latter can stabilize.
Desmosomal components present in the NP40-soluble fraction represent materials that are en route for either incorporation into desmosomes or degradation. Our finding of small amounts of tyrosine-phosphorylated Dsg2 and Pg in the soluble fraction of control cells suggests that tyrosine phosphorylation may be normally involved in one or other of these processes. Results from pervanadate-treated cells did not give a clear indication of whether an involvement in degradation or desmosomes assembly is more likely. We consistently found slightly less Dsg2 in the soluble fraction of treated cells than in controls, whereas the amount in the insoluble fraction remained constant. This might suggest an involvement in Dsg2 degradation since there was no evidence for increased incorporation into the insoluble fraction. On the other hand, the amount of Pg that co-precipitated with Dsg2 remained constant between control and treated cells, suggesting that tyrosine phosphorylation did not cause disruption of the Dsg2-Pg complex, but if anything enhanced it.
We conclude that the involvement of tyrosine phosphorylation in regulation of desmosomal adhesion is complex. On the one hand activation of EGF signalling destabilizes desmosomes; on the other, pervanadate treatment stabilizes them. Further work will be required in order to elucidate the subtleties of the mechanisms involved in these opposite responses to tyrosine phosphorylation of desmosomal components. Because of the involvement of desmosomes in human disease, including cancer27–29 and in morphogenesis30,31 such investigations are strongly merited.
Materials and Methods
Cell culture.
MDCK cells lines were maintained in Dulbecco's Minimum Essential Medium (DMEM) supplemented with 10% v/v FCS, 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C under 5% humidified CO2. Transfected cells were cultured in the presence of 500 µg/ml G418 to maintain selective pressure. To obtain subconfluent cultures cells were plated at a density of 1.25 × 104 cells.cm−2 and cultured for two days. (Under these conditions cells reached confluence after four days.) All experiments were performed on cells used within a space of 20 passages.
Transfection.
The plasmid was constructed by cutting pC-E-cadHA (a generous gift from Dr. Masayuki Ozawa) with Not I/EcoRV to remove the existing murine E-cadherin sequence, and ligating with NotI/EcoRV-digested PCR-generated full length mDsg2 (GenBank accession number AB072269). In order to detect expression in cells a HA tag sequence was added to their C-terminus. The resulting plasmid was confirmed by DNA sequencing and was termed pC-mDsg2HA. Transfection was carried out using Lipofectamine 2000 (Invitrogen) according to the manufacture instructions. Three days after transfection, the culture medium was changed to DMEM/10% FBS supplemented with 0.1 mg/ml G418 for selection. G418-resistant clones were isolated two weeks after transfection and screened by immunofluorescence and western blotting of cell lysates with anti-HA antibody.
Pervanadate preparation and treatment.
Pervanadate at 100 µmol dm−3 was prepared by incubating equal volumes of 100 mmol dm−3 Na3VO4 (pH 10) and fresh 30% v/v H2O2 solution for 15 minutes at room temperature before being diluted 1/500 in DMEM (without serum and antibiotics). This was used immediately to replace the cell culture medium and the dish placed in the incubator for 15 minutes before rinsing and preparation of cell extracts or fixation and processing for immunofluorescence. 100 mmol dm−3 Na3VO4 (pH 10) was prepared from powdered stock by making a 100 mmol dm−3 solution in water that was then adjusted to pH 10 at which point the solution became pale yellow. The solution was then boiled until colorless, allowed to cool and the pH measured and adjusted back to 10. The solution was then boiled until colorless again, allowed to cool and the pH measured to ensure it remained at 10. It was then aliquoted and stored at −20°C.
Antibodies.
Monoclonal antibodies 33-3D against the cytoplasmic domain of Dsg2 and 11-5F against the C-terminus of DP have been described previously.22–24 Monoclonal antibody PY99 against phosphotyrosine was from Santa Cruz and monoclonal antibody against Pg from Sigma. Monoclonal anti-HA antibody clone 12CA5 that was a generous gift from Professor Iain Hagan and polyclonal anti-HA was obtained from Sigma. No qualitative or quantitative differences between the IP characteristics of these two antibodies were observed.
Immunofluorescence.
Cells were cultured in 3.5 cm diameter dishes and fixed by removing the culture medium, replacing it immediately with methanol and incubating at −20°C for 5 minutes. All subsequent incubations were at room temperature. The methanol was diluted and the dishes rinsed three times with phosphate-buffered saline (PBS). The dishes were then blocked with PBS containing 5% v/v FCS for 30 minutes before incubation for 90 minutes with the manufacturer's recommended concentration of primary antibodies diluted in PBS containing 1% v/v FCS or with monoclonal antibody in hybridoma culture medium. Dishes were then rinsed thoroughly with PBS and incubated with fluorophore-conjugated anti-isotype-specific pre-adsorbed secondary antibodies diluted (always 1:100) in PBS containing 1% v/v FCS for 45 minutes in the dark. Nuclei of cells were visualized by subsequent incubation for 5 minutes with PBS containing Hoescht stain (bis-benzamidine). Dishes were then rinsed extensively with PBS and mounted under Vectashield™ with glass coverslips (grade 0). Fluorescence microscopy was carried out on a Zeiss Axioplan microscope using a planapochromat objective (63X/NA1.40) and images acquired using Metamorph software. Confocal microscopy was carried out on a Leica SP2 microscope using a AOBS lens (63x/NA1.40) with oil immersion at room temperature. Images were acquired and analyzed with Leica confocal software.
SDS-PAGE and immunoblotting.
Tris-glycine slab gels were cast using the Biorad Mini-protean II™ system. SDS-PAGE running buffer was 25 mmol dm−3 Tris pH 8.3, 192 mmol dm−3 glycine, 0.1% w/v SDS. Gels were typically run at 120 V for approximately 2 hours. A Biorad wet-transfer gel tank was used to electrotransfer proteins to nitrocellulose membranes by overnight wet blotting in transfer buffer (48 mmol dm−3 Tris pH 8.3, 39 mmol dm−3 glycine, 20% v/v methanol) (20 V for ∼16 hours). After transfer the membranes were blocked by a 30 minute incubation in 5% w/v milk/0.1% v/v Tween/Tris-buffered saline (TBS) (TBSTM). Membranes were then incubated overnight at 4°C or 90 minutes at room temperature with primary antibodies appropriately diluted in TBSTM. After extensive washing with 0.1% Tween/TBS (TBST) the membranes were incubated with horse radish peroxidase (HRP)-conjugated secondary antibodies diluted 1:8000 in TBSTM for 45 minutes at room temperature. The membrane was extensively washed with TBST and immunoreactive bands visualized by immersing the membrane in ECL reagent for 2 minutes prior to wrapping in transparent cling-film and exposure to light-sensitive film.
Cell fractionation and immunoprecipitation.
Three 10 cm diameter dishes were used for each sample. Cultures expressing ectopic HAmDSG2 were placed on ice and washed x2 with ice-cold PBS before the addition of 750 µl of ‘dilution buffer’ [25 mmol dm−3 HEPES pH 7.5, 150 mmol dm−3 NaCl, 4 mmol dm−3 EDTA, 50 mmol dm−3 β-glycerophosphate, 0.5 mmol dm−3 PMSF, 10 µg/ml aprotinin, 10 µg/ml leupeptin, 25 mmol dm−3 NaF, 1 mmol dm−3 Na3VO4, 1% v/v IGEPAL CA-530 (NP-40)] and incubated for 15 minutes at 4°C on a rocking platform.
Dishes were then scraped with a fresh rubber cell scraper and collected in chilled microcentrifuge tubes before centrifugation at 12,000 × g for 10 minutes. The supernatant (the ‘soluble fraction’) was then removed and the pellet resuspended in 75 µl of boiling ‘SDS buffer’ (25 mmol dm−3 HEPES pH 7.5, 4 mmol dm−3 EDTA, 25 mmol dm−3 NaF, 1 mmol dm−3 Na3VO4, 1% w/v SDS) before being heated to 95°C for 10 minutes. A nine-fold volume (675 µL) of ice-cold ‘dilution buffer’ was then added and the sample passed through a fresh 27G syringe 10 times before being centrifuged as above and the supernatant (the ‘insoluble fraction’) retained as the immunoprecipitation (IP) sample. Samples were pre-cleared with a 20 µl bed volume of protein G-Sepharose prior to the addition of 2 µg of monoclonal anti-HA antibody and rotation for 3 hours at 4°C. The pre-clearing beads were washed x3 with ‘dilution buffer’, eluted into fresh 5x Laemmli buffer (250 mmol dm−3 Tris pH 6.8, 50% v/v glycerol, 2.5% w/v bromophenol blue, 20 mmol dm−3 DTT) and used as a negative control for the IP. Immune complexes were captured by addition of a 20 µl bed-volume of protein-G Sepharose and further rotation for 40 minutes at 4°C. The beads were then washed x3 with ‘dilution buffer’ and eluted into 5x Laemmli buffer.
Acknowledgements
We thank Dr. David Wateridge for technical assistance and Drs. Lydia Tabernero and Tomomi Kimura for helpful comments on the manuscript. The work was supported by the Medical Research Council.
Abbreviations
- PKC
protein kinase C
- EGF
epidermal growth factor
- Dp
desmoplakin
- Dsg
desmoglein
- Pg
plakoglobin
- LCM
low calcium medium
- MDCK
Madin-Darby canine kidney
- PMA
phorbol myristate acetate
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/6549
References
- 1.Garrod D, Chidgey M. Desmosome structure, composition and function. Biochim Biophys Acta. 2008;1778:572–587. doi: 10.1016/j.bbamem.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Kimura TE, Merritt AJ, Garrod DR. Calcium-independent desmosomes of keratinocytes are hyper-adhesive. J Invest Dermatol. 2007;127:775–781. doi: 10.1038/sj.jid.5700643. [DOI] [PubMed] [Google Scholar]
- 3.Green KJ, Simpson CL. Desmosomes: new perspectives on a classic. J Invest Dermatol. 2007;127:2499–2515. doi: 10.1038/sj.jid.5701015. [DOI] [PubMed] [Google Scholar]
- 4.Wallis S, Lloyd S, Wise I, Ireland G, Fleming TP, Garrod D. The alpha isoform of protein kinase C is involved in signaling the response of desmosomes to wounding in cultured epithelial cells. Mol Biol Cell. 2000;11:1077–1092. doi: 10.1091/mbc.11.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garrod DR, Berika MY, Bardsley WF, Holmes D, Tabernero L. Hyper-adhesion in desmosomes: its regulation in wound healing and possible relationship to cadherin crystal structure. J Cell Sci. 2005;118:5743–5754. doi: 10.1242/jcs.02700. [DOI] [PubMed] [Google Scholar]
- 6.Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irby RB, Yeatman TJ. Increased Src activity disrupts cadherin/catenin-mediated homotypic adhesion in human colon cancer and transformed rodent cells. Cancer Res. 2002;62:2669–2674. [PubMed] [Google Scholar]
- 8.Volberg T, Geiger B, Dror R, Zick Y. Modulation of intercellular adherens-type junctions and tyrosine phosphorylation of their components in RSV-transformed cultured chick lens cells. Cell Regul. 1991;2:105–120. doi: 10.1091/mbc.2.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeda H, Nagafuchi A, Yonemura S, Tsukita S, Behrens J, Birchmeier W, Tsukita S. V-src kinase shifts the cadherin-based cell adhesion from the strong to the weak state and beta catenin is not required for the shift. J Cell Biol. 1995;131:1839–1847. doi: 10.1083/jcb.131.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avizienyte E, Wyke AW, Jones RJ, McLean GW, Westhoff MA, Brunton VG, Frame MC. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling. Nat Cell Biol. 2002;4:632–638. doi: 10.1038/ncb829. [DOI] [PubMed] [Google Scholar]
- 11.Jones RJ, Avizienyte E, Wyke AW, Owens DW, Brunton VG, Frame MC. Elevated c-Src is linked to altered cell-matrix adhesion rather than proliferation in KM12C human colorectal cancer cells. Br J Cancer. 2002;87:1128–1135. doi: 10.1038/sj.bjc.6600594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cartwright CA, Meisler AI, Eckhart W. Activation of the pp60c-src protein kinase is an early event in colonic carcinogenesis. Proc Natl Acad Sci USA. 1990;87:558–562. doi: 10.1073/pnas.87.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cartwright CA, Kamps MP, Meisler AI, Pipas JM, Eckhart W. pp60c-src activation in human colon carcinoma. J Clin Invest. 1989;83:2025–2033. doi: 10.1172/JCI114113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolen JB, Veillette A, Schwartz AM, DeSeau V, Rosen N. Activation of pp60c-src protein kinase activity in human colon carcinoma. Proc Natl Acad Sci USA. 1987;84:2251–2255. doi: 10.1073/pnas.84.8.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irby RB, Mao W, Coppola D, Kang J, Loubeau JM, Trudeau W, Karl R, Fujita DJ, Jove R, Yeatman TJ. Activating SRC mutation in a subset of advanced human colon cancers. Nat Genet. 1999;21:187–190. doi: 10.1038/5971. [DOI] [PubMed] [Google Scholar]
- 16.Taddei ML, Chiarugi P, Cirri P, Buricchi F, Fiaschi T, Giannoni E, Talini D, Cozzi G, Formigli L, Raugei G, Ramponi G. Beta-catenin interacts with low-molecular-weight protein tyrosine phosphatase leading to cadherin-mediated cell-cell adhesion increase. Cancer Res. 2002;62:6489–6499. [PubMed] [Google Scholar]
- 17.Gaudry CA, Palka HL, Dusek RL, Huen AC, Khandekar MJ, Hudson LG, Green KJ. Tyrosine-phosphorylated plakoglobin is associated with desmogleins but not desmoplakin after epidermal growth factor receptor activation. J Biol Chem. 2001;276:24871–24880. doi: 10.1074/jbc.M102731200. [DOI] [PubMed] [Google Scholar]
- 18.Yin T, Getsios S, Caldelari R, Godsel LM, Kowalczyk AP, Muller EJ, Green KJ. Mechanisms of plakoglobin-dependent adhesion: desmosome-specific functions in assembly and regulation by epidermal growth factor receptor. J Biol Chem. 2005;280:40355–40363. doi: 10.1074/jbc.M506692200. [DOI] [PubMed] [Google Scholar]
- 19.Lorch JH, Klessner J, Park JK, Getsios S, Wu YL, Stack MS, Green KJ. Epidermal growth factor receptor inhibition promotes desmosome assembly and strengthens intercellular adhesion in squamous cell carcinoma cells. J Biol Chem. 2004;279:37191–37200. doi: 10.1074/jbc.M405123200. [DOI] [PubMed] [Google Scholar]
- 20.Miravet S, Piedra J, Castano J, Raurell I, Franci C, Dunach M, Garcia de Herreros A. Tyrosine phosphorylation of plakoglobin causes contrary effects on its association with desmosomes and adherens junction components and modulates beta-catenin-mediated transcription. Mol Cell Biol. 2003;23:7391–7402. doi: 10.1128/MCB.23.20.7391-7402.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozawa M, Kemler R. Altered cell adhesion activity by pervanadate due to the dissociation of alpha-catenin from the E-cadherin.catenin complex. J Biol Chem. 1998;273:6166–6170. doi: 10.1074/jbc.273.11.6166. [DOI] [PubMed] [Google Scholar]
- 22.Parrish EP, Steart PV, Garrod DR, Weller RO. Antidesmosomal monoclonal antibody in the diagnosis of intracranial tumors. J Pathol. 1987;153:265–273. doi: 10.1002/path.1711530311. [DOI] [PubMed] [Google Scholar]
- 23.Vilela MJ, Hashimoto T, Nishikawa T, North AJ, Garrod D. A simple epithelial cell line (MDCK) shows heterogeneity of desmoglein isoforms, one resembling pemphigus vulgaris antigen. J Cell Sci. 1995;108:1743–1750. doi: 10.1242/jcs.108.4.1743. [DOI] [PubMed] [Google Scholar]
- 24.North AJ, Bardsley WG, Hyam J, Bornslaeger EA, Cordingley HC, Trinnaman B, Hatzfeld M, Green KJ, Magee AI, Garrod DR. Molecular map of the desmosomal plaque. J Cell Sci. 1999;112:4325–4336. doi: 10.1242/jcs.112.23.4325. [DOI] [PubMed] [Google Scholar]
- 25.Calautti E, Grossi M, Mammucari C, Aoyama Y, Pirro M, Ono Y, Li J, Dotto GP. Fyn tyrosine kinase is a downstream mediator of Rho/PRK2 function in keratinocyte cell-cell adhesion. J Cell Biol. 2002;156:137–148. doi: 10.1083/jcb.200105140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amino K, Takahashi M, Honda Y, Fujimoto T. Tyrosine phosphorylation in desmosomes and hemidesmosomes of the corneal epithelium. J Histochem Cytochem. 1996;44:19–25. doi: 10.1177/44.1.8543777. [DOI] [PubMed] [Google Scholar]
- 27.Chidgey M. Desmosomes and disease: an update. Histol Histopathol. 2002;17:1179–1192. doi: 10.14670/HH-17.1179. [DOI] [PubMed] [Google Scholar]
- 28.Chidgey M, Dawson C. Desmosomes: a role in cancer? Br J Cancer. 2007;96:1783–1787. doi: 10.1038/sj.bjc.6603808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kottke MD, Delva E, Kowalczyk AP. The desmosome: cell science lessons from human diseases. J Cell Sci. 2006;119:797–806. doi: 10.1242/jcs.02888. [DOI] [PubMed] [Google Scholar]
- 30.Cheng X, Den Z, Koch PJ. Desmosomal cell adhesion in mammalian development. Eur J Cell Biol. 2005;84:215–223. doi: 10.1016/j.ejcb.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Runswick SK, O'Hare MJ, Jones L, Streuli CH, Garrod DR. Desmosomal adhesion regulates epithelial morphogenesis and cell positioning. Nat Cell Biol. 2001;3:823–830. doi: 10.1038/ncb0901-823. [DOI] [PubMed] [Google Scholar]