Abstract
The central nervous system (CNS) acquires its vasculature by angiogenesis, a process consisting of proliferation of endothelial cells in existing blood vessels or vascular plexuses and leading to formation of new blood vessels. Angiogenesis begins early in CNS development and continues throughout life. Prevailing notions depict CNS angiogenesis as a passive process driven primarily by demands for oxygen and other nutrients by the growing neuronal populations. Thus, although the CNS vasculature develops concomitant with neuronal identities, which emerge under the influence of compartment-specific cell autonomous factors, cell autonomous patterning signals are not considered to instruct vascular development. We have challenged this prevailing notion by showing that angiogenesis in the mouse telencephalon progresses in an orderly, ventral-to-dorsal gradient regulated in a cell-autonomous manner by compartment-specific homeobox transcription factors. These are the same transcription factors that confer compartmental identities on telencephalic neurons and progenitor populations. Thus, the same cell-autonomous, regional patterning signals that regulate development of telencephalic neuronal networks also regulate development of telencephalic vascular networks, underscoring shared mechanisms in CNS vascular and neuronal development. These novel concepts represent a new twist in the intriguing tale of CNS angiogenesis and offer new perspectives on telencephalic regionalization and histogenesis principles.
Key words: CNS, angiogenesis, homeobox, transcription factor, migration
The anatomy of the brain's vascular networks is just as complex and fascinating as that of its neuronal networks. Yet, despite the remarkable progress in our understanding of the mechanisms of development of neuronal networks, the mechanisms regulating CNS angiogenesis are not well understood. The generation of blood vessels in the mammalian embryo begins with a process of vasculogenesis when a subset of splanchnopleuric mesodermal cells give rise to blood islands. The cells at the periphery of the blood islands are endothelial cell precursors called angioblasts whereas cells at the center are hematopoietic precursor cells.1 New blood vessels sprout from the blood islands by angiogenesis, which is a process consisting of endothelial cell proliferation and sprouting and migration of new vessels.
In the mouse, the first blood islands arise around embryonic day 7 (E7).2 Some blood islands migrate to the head region of the embryo to produce cranial vessels, including blood vessels of the brain. Until recently, development of blood vessels of the brain was considered to be a passive process occurring secondarily in response to metabolic demands of the growing brain tissue.3,4 Cell autonomous mechanisms that regulate development of the other cell type in the brain, the neuron, were not considered to apply to the development of endothelial cells, building blocks of blood vessels. This concept appeared rather curious to us because blood vessels develop concomitant with neuronal networks, and in a molecular milieu replete with cues that guide neuronal development. We investigated the pattern and mechanisms regulating development of the blood vessels in the embryonic mouse telencephalon (forebrain) and our data paint a picture of angiogenesis in the embryonic brain that is strikingly different from the notion that CNS angiogenesis is a passive process merely shadowing neuronal development. We show that brain's blood vessels develop according to an intrinsic program guided by some of the same cell autonomous factors that guide neuronal development.5
Blood vessels in the embryonic mouse brain fall into two categories based on anatomical location, growth patterns and developmental regulation: pial vessels and periventricular vessels. The pial vessels encompass the entire brain as early by E9, without any apparent spatial or temporal gradient in their development. The periventricular vessels, on the other hand, develop in an orderly, ventral-to-dorsal gradient forming a lattice, resembling a diamond necklace encircling the ventricular cavities and situated half way between the ventricular and pial surfaces (Fig. 1). The periventricular vessels are branches of a basal vessel located in the basal ganglia primordium that likely arises from the pharyngeal arch arteries located in the cervical region. An elegant study using scanning electron microscopy of vascular corrosion casts and 3D reconstruction of serial 1 µm-thick sections to study the arterial network of the embryonic mouse brain confirms the origin of the periventricular vessel network from the basal vessel.6 It is likely that the periventricular vessels develop into arterial networks and pial vessels into venous sinuses.6
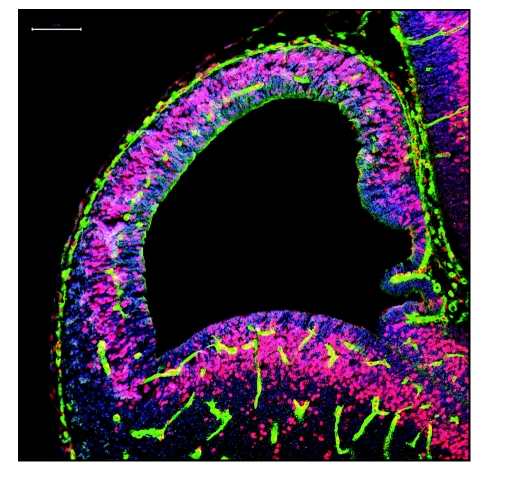
Figure 1.
Immunohistochemical labeling for blood vessels (Isolectin B4-green), bromodeoxyuridine (BrdU, 2.0 hr exposure, red/pink) and the stem/progenitor cell marker Musashi (blue) in a 20 µm-thick coronal paraffin section through E11 mouse telencephalon. Pial and periventricular vessels in the dorsal and ventral telencephalon show many BrdU-labeled nuclei. The periventricular vessel developmental gradient is apparent in the dorsal telencephalon: the vessels have not yet arrived at the medial pole (the septo-hippocampal primordium) while the pial vessels encircle the entire telencephalon. Scale bar: 100 µm.
Our finding that the periventricular vessels are the major population of vessels in the parenchyma of the embryonic telencephalon and that these vessels developed in a spatial-temporal gradient underscored interesting concepts. The direction of propagation of the periventricular angiogenesis gradient matches that of the telencephalic transverse neurogenetic gradient,7,8 although the angiogenesis gradient is in advance of the neurogenetic gradient temporally,5 by about a day. By E11, the periventricular vessels invade the dorsal telencephalon in a lattice pattern, approaching its medial wall and establish a ventro-lateral to dorso-medial angiogenesis gradient (Fig. 1). Within the dorsal telencephalon, the periventricular vessel gradient and the gradient of appearance of GABA neurons overlap spatially as well. Here too, the angiogenesis gradient is in advance of the GABA neuron gradient temporally. These observations raised an interesting question: Are the angiogenesis, neurogenesis and GABA neuron developmental gradients in the telencephalon related to one another at a mechanistic level?
Telencephalic neurogenesis and GABA neuron migration are regulated by region-specific homeobox transcription factors, which confer cell-autonomous regional identities upon neurons and their precursor cells.9–11 In mice with mutations or deletions of the transcription factor genes Nkx2.1, Dlx1&2 and Pax6, there is significant impairment of neurogenesis and a re-patterning of the telencephalic compartments.12 We found that the telencephalic periventricular angiogenesis gradient is impaired in mice lacking these transcription factors.5 The loss of the transcription factors decreased periventricular endothelial cell proliferation and migration in a compartment-specific and cell autonomous fashion. By using heterochronic transplantation and siRNA gene knock-down assays, we showed that the disruption of the angiogenesis gradient, endothelial cell proliferation and migration were not secondary consequences of impaired tissue oxygenation, neurogenesis, neuronal migration or differentiation: these effects were a direct result of the loss of the transcription factor genes in the endothelial cells.5 A diagrammatic representation of the angiogenesis gradient in the embryonic mouse telencephalon and its relationship to homeobox transcription factor expression domains is shown (Fig. 2).
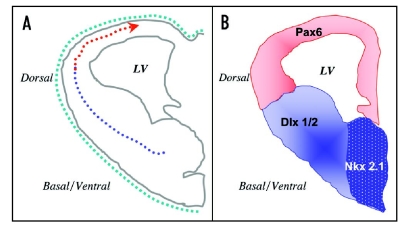
Figure 2.
Diagrammatic illustration of the angiogenesis gradient in the embryonic mouse telencephalon and its relationship to homeobox transcription factor expression domains. (A) The ventral-to-dorsal and lateral-to-medial gradient of periventricular angiogenesis (broken line with directional arrow) is illustrated in a transverse section through embryonic day 11 (E11) telencephalic hemisphere. Pial vessels, which develop earlier and encircle the entire telencephalon by E9 without any developmental gradient, are shown as a green broken line. Thus, the telencephalic angiogenesis gradient is a gradient in the formation of periventricular vessels and it is established by migration of endothelial cells across telencephalic compartments (in the direction of the red arrow). Periventricular endothelial cells in the ventral (blue broken line) show distinct transcription factor expression profiles compared to those in the dorsal telencephalon (red broken line) corresponding to the domains of expression of compartment-specific transcription factors shown in (B). (B) Periventricular endothelial cells in the ventral telencephalon express ventral transcription factors Dlx1/2 and Nkx2.1 while those in the dorsal telencephalon express Pax6. Loss of these transcription factors perturbs endothelial cell development in a region-specific manner. The stippled blue region represents overlapping expression domains of Dlx1/2 and Nkx2.1.
Thus development of endothelial cells in the brain appears to be regulated by some of the same mechanisms that regulate patterning of telencephalic compartments in general, suggesting shared mechanisms in the regulation of endothelial and neuronal development. In fact, a review of the literature indicates that an overlapping repertoire of signalling molecules control angiogenesis and neurogenesis in the developing brain.13–15 Molecules produced in one system influence development of the other by promoting proliferation, differentiation or process outgrowth. Cell sorting and migration in both vascular and neural systems rely on the use of similar classes of molecules that attract cells of common origins or function (cadherins) and establish boundaries between cells of distinct molecular properties (neuropilins, semaphorins).16–18
Although endothelial cell development in the embryonic telencephalon is regulated by cell-intrinsic factors that also regulate neuronal development, is endothelial cell development regulated independently of neuronal development? Can selective impairment of endothelial cell development have consequences for telencephalic neuronal development? Finally, how do these transcription factors regulate endothelial cell development?
The migrating periventricular endothelial cells and GABA neurons migrating from the basal to the dorsal telencephalon follow the same migratory route. Since the endothelial cells migrate at least a day in advance of the GABA neurons, it is tempting to speculate that the endothelial cell migratory stream may lay down a permissive tract for the migration of the GABA neurons. The endothelial cell migratory stream may also facilitate cortico-fugal and thalamo-cortical axon pathfinding because these axonal pathways also follow the route laid down a few days earlier by the endothelial cell migratory stream. In addition, the dynamic up or downregulation of gene expression accompanying cross-border migration of the periventricular endothelial cells5 render these cells ideal for morphogen synthesis, delivery and establishment of morphogenetic gradients. That endothelial cells can mediate morphogen gradients is a novel idea in developmental neurobiology and is likely to offer new insights into the mechanisms governing the establishment and propagation of morphogenetic gradients in the developing CNS. If endothelial cells can influence GABA neuron migration, cortical axon pathfinding or morphogenetic gradients and since endothelial cell migration precedes all of these other processes, it seems reasonable to suggest that impairment of endothelial cell development can significantly impair histogenesis of the telencephalon.
The preceding observations suggest that a search for genetic or environmental factors that target endothelial cell development in the embryonic brain will be a worthwhile endeavor for understanding the etiology of developmental CNS disorders. Along the same lines, since the endothelial cells secrete a variety of growth factors and guidance cues, implantation of endothelial cells into damaged brain regions may facilitate stereotyped guidance of vessels, neurons or nerve fibers aiding in recovery of neurological function in stroke, traumatic brain injury or neurodegeneration. Further research into the mechanisms governing CNS angiogenesis and the role of endothelial cells in sculpting neuronal networks may lead to discoveries with unprecedented implications for understanding and treatment of neurological disorders.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/6485
References
- 1.Risau W, Flame L. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 2.Patan S. Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J Neurooncol. 2000;50:1–15. doi: 10.1023/a:1006493130855. [DOI] [PubMed] [Google Scholar]
- 3.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 4.Kurz H. Physiology of angiogenesis. J Neurooncol. 2000;50:17–35. doi: 10.1023/a:1006485716743. [DOI] [PubMed] [Google Scholar]
- 5.Vasudevan A, Long JE, Crandall JE, Rubenstein JL, Bhide PG. Compartment-specific transcription factors orchestrate angiogenesis gradients in the embryonic brain. Nat Neurosci. 2008;11:429–439. doi: 10.1038/nn2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiruma T, Nakajima Y, Nakamura H. Development of pharyngeal arch arteries in early mouse embryo. J Anat. 2002;201:15–29. doi: 10.1046/j.1469-7580.2002.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayer SA, Altman J. Directions in neurogenetic gradients and patterns of anatomical connections in the telencephalon. Prog Neurobiol. 1987;29:57–106. doi: 10.1016/0301-0082(87)90015-3. [DOI] [PubMed] [Google Scholar]
- 8.Bayer SA, Altman J. Neocortical Development. New York: Raven Press; 1991. [Google Scholar]
- 9.Puelles L, Rubenstein JLR. Expression patterns of homeobox and other putative regulatory genes in the embryonic mouse forebrain suggests a neuromeric organization. TINS. 1993;16:472–479. doi: 10.1016/0166-2236(93)90080-6. [DOI] [PubMed] [Google Scholar]
- 10.Rubenstein JL, Anderson S, Shi L, Miyashita-Lyn E, Bulfone A, Hevner R. Genetic control of cortical regionalization and connectivity. Cereb Cortex. 1999;9:524–532. doi: 10.1093/cercor/9.6.524. [DOI] [PubMed] [Google Scholar]
- 11.Anderson SA, Mione MC, Yun K, Rubenstein JLR. Differential origins of neocortical projection and local circuit neurons: Role of Dlx genes in neocortical interneuronogenesis. Cereb Cortex. 1999;9:646–654. doi: 10.1093/cercor/9.6.646. [DOI] [PubMed] [Google Scholar]
- 12.Marin O, Rubenstein JL. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2:780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet P. Blood vessels and nerves: common signals, pathways and diseases. Nat Rev Genet. 2003;4:710–720. doi: 10.1038/nrg1158. [DOI] [PubMed] [Google Scholar]
- 14.Haigh JJ, Morelli PI, Gerhardt H, Haigh K, Tsien J, Damert A, Miquerol L, Muhlner U, Klein R, Ferrara N. Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev Biol. 2003;262:225–241. doi: 10.1016/s0012-1606(03)00356-7. [DOI] [PubMed] [Google Scholar]
- 15.Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 16.Marin O, Yaron A, Bagri A, Tessier-Lavigne M, Rubenstein JL. Sorting of striatal and cortical interneurons regulated by semaphorin-neuropilin interactions. Science. 2001;293:872–875. doi: 10.1126/science.1061891. [DOI] [PubMed] [Google Scholar]
- 17.Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999;126:4895–4902. doi: 10.1242/dev.126.21.4895. [DOI] [PubMed] [Google Scholar]
- 18.Kawakami A, Kitsukawa T, Takagi S, Fujisawa H. Developmentally regulated expression of a cell surface protein, neuropilin, in the mouse nervous system. J Neurobiol. 1996;29:1–17. doi: 10.1002/(SICI)1097-4695(199601)29:1<1::AID-NEU1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]