Abstract
Atherosclerosis is currently the leading factor of death in developed countries. It is now recognized as a chronic immune-inflammatory disease, whose initial stages involve the interaction of leukocytes with the endothelial monolayer. The initial stage of atherosclerosis requires the interplay of various cell adhesion molecules and immune cells to trigger leukocyte and lymphocyte migration from the circulating blood into the arterial intima. Studies have unveiled the role of inflammatory mediators in the initiation, onset and progression of the disease. During the last few years we have gained a greater understanding of the mechanism that modulates monocyte, macrophage and T cell infiltration, the role these cells play in the atherosclerotic lesion, in the formation of the fibrous plaque formation with the consequent narrowing of the arteries and the mechanisms that lead to plaque rupture and the formation of thrombi and emboli. This review talks about the leukocyte recruitment in early atherosclerosis, the formation of the plaque, and the mechanisms that lead to thrombosis in advanced atherosclerosis. Finally, we discuss the potential for novel therapies to treat this disease.
Key Words: CAMs, leukocyte, lymphocyte, migration, atherosclerosis, extravasation
Introduction
Atherosclerosis, currently a leading factor of heart disease and stroke, causes almost 50% of all deaths every year in developed countries. It has recently been appreciated that atherosclerosis is a chronic disease, related to immune-inflammation, where the interaction of immune cells with blood vessel endothelium is a crucial event. It is characterized by the accumulation of lipoproteins and fibrous elements in large arteries.1 Mice models used in physiological studies have shown that development of atherosclerosis can fall into three stages.2 The first obvious change, observed at the initiation of plaque formation, is the accumulation and aggregation of lipoprotein particles, at the preferential lesion-sites in the intima, following a continuous feeding of a high-cholesterol diet.3 A few days later, monocytes can adhere and migrate across the endothelial layer, through the mechanism of extravasation, and there they will differentiate into macrophages and will take up lipoproteins, causing the formation of foam cells.4 The lipid contents from dead foam cells will contribute to the necrotic core of the lesion: accumulating smooth muscle cells secreting fibrous elements will also contribute to increase the size of the fibrous plaque.2 The lesion continues to grow by the migration of mononuclear cells, from the blood stream, into the shoulder region of the vessel, which is accompanied by cell proliferation and extracellular matrix formation.3 It is said that atherosclerosis can be regarded as a ‘response to injury’ and the injurious agents are the lipoproteins.3–5
Lesion Initiation
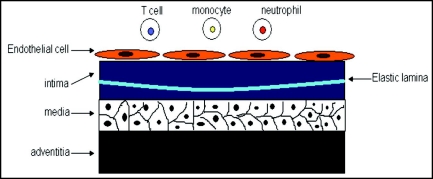
The artery wall consists of three major distinct layers, the intima, the media and the adventitia.3 The intima is the innermost layer covered by a monolayer of endothelial cells on the luminal side and a sheet of internal elastic lamina on the peripheral side. The media is rich in smooth muscle cells, and the adventitia consists of connective tissues.3 This structure is summarized in Figure 1. The regions of arterial branching, where the flow is disturbed, show increased permeability to macromolecules, such as low-density lipoprotein (LDL) and they are sites of lesion predilection.6 Accumulation of LDL in the endothelial layer is the primary event of atherosclerosis.2–4 LDL can diffuse into the subendothelial matrix through cell junctions and one major constituent of LDL, apolipoprotein B (apo B), interacts with the matrix proteoglycans in the endothelial monolayer.7 It is shown that only modified LDL can be taken by macrophages to form foam cells.8 It is believed that accumulated LDL undergoes oxidation, lipolysis, proteolysis or aggregation and that oxidation is the most significant modification for lesion initiation.2 With regards to high-density lipoprotein, it can prevent atherosclerosis to a certain extent. It plays an important role in the removal of excess cholesterol, as well as inhibiting lipoprotein oxidation.3
Figure 1.
The structure of the large artery consists of three major distinct layers: the intima, the media and the adventitia. The intima is the innermost layer, covered by a monolayer of endothelial cells on the luminal side and a sheet of internal elastic lamina on the peripheral side. The media is rich in smooth muscle cells and the adventitia consists of connective tissues.
Recruitment of Leukocytes
Cell adhesion and migration.
In an inflammatory response, cell adhesion is followed by leukocyte transmigration through the endothelial layer into the intima.9,10 The accumulated oxidized LDL (oxLDL) can activate the endothelial cells to express adhesion molecules, which are indispensable in the blood cell recruitment.11 The first step of ‘adhesion’ is the tethering and rolling of blood cells along the surface of the endothelial monolayer, which is mediated by the selectin family of adhesion proteins.12 P- and E-selectin are expressed on the surface of the activated endothelium and they bind to carbohydrate ligands on leukocytes.9 Mice that lack P- and E-selectin, and that were induced to develop atherosclerosis, showed a profound decrease in atherosclerosis, indicative of the role of these molecules in the disease pathogenesis.10 The firm attachment of monocytes and T cells to the endothelium is mediated by the interaction of intercellular adhesion molecule1 (ICAM1) or vascular cell adhesion molecule1 (VCAM1) and integrin VLA-4 on the endothelium and the monocytes, respectively.9,10,13 It is also believed that other receptors, such as Fc receptors, expressed by activated monocytes may facilitate their adhesion to the endothelial cells.13 Both in vivo and in vitro studies have shown the role of the different interactions between these different cell adhesion molecules in atherosclerosis.14
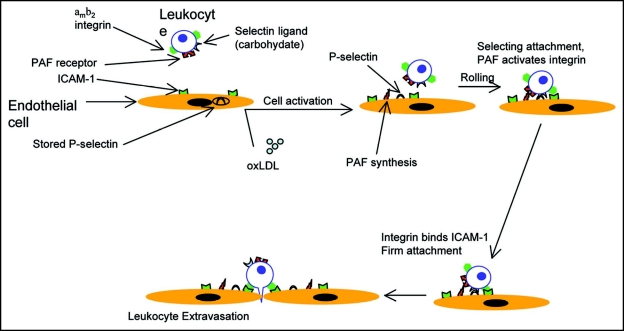
Interestingly, integrins are expressed on leukocytes in a fashion that is of low affinity to ligands, and they undergo activation by cellular activation, mediated by proinflammatory molecules.15 Therefore, in addition to surface adhesion molecules, multiple chemokines and cytokines regulate the adhesion of blood cells to the endothelial monolayer of the arterial wall and also guide the leukocytes migration.16 Studies using CCL2-/- and/or CCR2-/- mice show that the CC-chemokine ligand 2 (CCL2 or MCP1) and the receptor, CC-chemokine receptor 2 (CCR2) play a key role in the entry of monocytes and T cells into the intima.17 Other cytokines and chemokines believed to play a role in immune-cell migration include macrophage colony-stimulating factor (M-CSF), granulocyte/macrophage colony-stimulating factor (GM-CSF), migratory inflammatory protein (MIP)-1, TGFβ and T-cell attractants CCL5.13 Overexpression of other chemokines such as CXC chemokines, induced by interferon-γ (IFNγ), also contributes to lymphocyte recruitment.18 The pro-inflammatory cytokines produced by cells in the intima, in response to lipoproteins, can activate monocytes in the blood stream. These include interleukin (IL)-1, -6, -8, -10, -12 and TNFα.18 oxLDL directly activates endothelial cells to stimulate monocyte migration from the blood stream into the intima. A number of reviews have highlighted that activated endothelial cells express various growth factors and mediators such as nitric oxide (NO) and angiotensins 1 and 2 which in turn regulate the adhesion properties of the endothelium.13,19,20 It is also known that the concentrations of platelet endothelial adhesion molecule (PECAM)-1 and junctional adhesion molecule-A (JAM-A) are very high at the cell-cell contact surfaces and they are found to be expressed on activated leukocytes.21 The process of monocyte extravasation is shown in Figure 2.
Figure 2.
Oxidized-LDL (oxLDL) triggers the upregulation of endothelial cell-adhesion-molecule expression, passing leukocytes and lymphocytes attach to endothelial cells via tethering to the CAMs. Selectins are CAMs specific for leukocyte-vascular interactions. P-selectin is stored in intracellular vesicles in unstimulated endothelial cells. Activation of endothelial cells results in the trafficking of the vesicles containing P-selectin to the cell surface. Surface expression of P-selecting forms week interactions with passing leukocytes, which are slowed but not stopped. For tight adhesion to occur β2 containing integrins must be activated. Activation of the αmβ2 integrin (expressed in monocytes), induced by PAF. Binding of PAF to its receptor on immune-cells activates the β2-integrins through Rho. Activated integrins then bind to ICAMs, expressed on the surface of endothelial cells, allowing tight adhesion.
Monocytes differentiation and T cell activation.
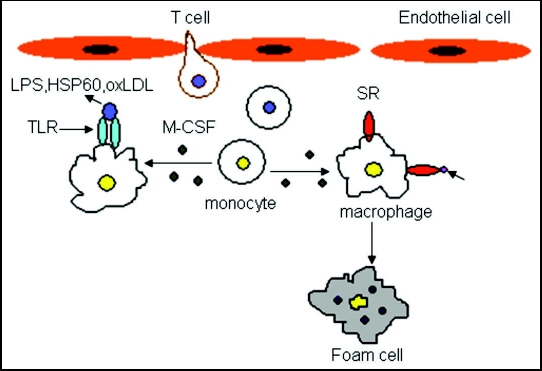
Monocytes will differentiate into macrophages after their entrapment in the intima. M-CSF is known to play a key role in stimulating the differentiation of monocytes into macrophages.22 Moreover, studies of knockout mice have shown that lack of M-CSF leads, to retarded lesion development and reduced macrophage accumulation.23,24 Expression of many pattern-recognition receptors increases on the surface of the newly differentiated macrophages, including scavenger receptors (SR, a family of proteins which includes CD36, CD68, CXCL16, SR-A and SR-B1) and Toll-like receptors (TLR).25 SR, present on macrophages, in the intima, can bind to and internalize oxLDL, leading to the formation of foam cells.25 TLR binds to lipopolysaccaride (LPS) or heat-shock protein 60 (HSP60) to trigger macrophages to produce pro-inflammatory molecules, such as cytokines, chemokines, matrix metalloproteinases (MMP) and nitric oxide (NO):26 this is summarized in Figure 3. Interestingly, monocytes not just differentiate into macrophages, but also into dendrictic cells (DC),27 this may lead to strong antigen presentation within the intima. The differentiation of monocytes into the different two lineages depends on the balance of M-CSF and GM-CSF.28 In the subendothelial layer, local antigens presented on the DCs and macrophages may interact with T cells and trigger the subsequent T cell activation.16 Inflammatory cytokines such as IFNγ and TNFα, produced by activated T cells, will also in turn instigate macrophage activation and the release of other mediators.16 It is proposed that the initial round of T cell activation may occur in the regional lymph nodes because the antigen-presenting cells may traffic from the plaque to the lymph nodes.29 After that, the activated T cells bind to adhesion molecules to enter the plaque, where macrophages might present antigens to these T cells, leading to their further rounds of activation.25
Figure 3.
Expression of many pattern-recognition receptors increases on the surface of the newly formed macrophages, such as scavenger receptors (SR, a family of proteins including CD36, CD68, CXCL16, SR-A and SR-B1) and Toll-like receptors (TLR). SR can bind to the oxLDL in the intima, leading to the formation of foam cells. T-cells migrate into the intima and secrete cytokines for monocyte differentiation.
Foam Cell Formation
Formation of foam cells.
In the arterial wall, macrophages contribute to the formation of fibrous plaque by metabolizing various subendothelial components.30 Lipoproteins infiltrate into the intima and get trapped before the modification occurs.31 Enzymatic or nonenzymatic oxidation, which results in the production of oxLDL, is the most common modification for LDLs. As discussed earlier, these modified lipoprotein aggregates will be internalized upon binding to the SRs expressed on the surface of macrophages, eventually leading to the formation of foam cells.3–5 Other than the reactive oxygen species produced by the ECs and macrophages, enzymes such as myeloperoxidase, sphingomyelinase and a secretory phospholipase A2 are all involved in LDL modification in human atherosclerotic lesions.32,33 Myeloperoxidase generates highly reactive oxygen species,32 sphingomyelinase accelerates lipoprotein aggregation33 and secretory phospholipase A2 promotes LDL oxidation.32 The expression of SRs is mediated by peroxisome proliferator-activated receptor-γ (PPAR) and cytokines such as IFNγ and TNFα.34 Macrophages can produce apoE, which inhibits the transition of macrophages into foam cells.35 Studied have shown that mice transplanted with bone marrow of apoE deficient mice develop larger atherosclerotic lesions than mice receiving marrows from normal mice.35
It has been noticed that in the early stage of atherosclerosis, the exit of some foam cells from plaque to the bloodstream may occur, but it is seldom seen in the late stage, when the fibrous cap forms.19 The transformation of macrophages to foam cells might be influenced by various factors other than just the ingestion of oxLDLs: it is suggested that immune complexes, as well as the peroxisome proliferator-activated receptor, play a role in foam cell formation.34
Formation of the necrotic core.
Intensive aggregation of the foam cells can lead to the formation of the necrotic core. It has been shown that E-cadherin expression is upregulated when foam cells are scattered but its expression is downregulated when foam cells aggregate.36 In the center of the core, the foam cells die and the debris accumulates. This is accompanied by the accumulation of extracellular lipids. Cell death may be due to the DNA damage caused by ox-LDL.37 It is widely considered that cell death at the core occurs through apoptosis, via the mediation of Fas, p53 or oxysterols;13,37 in contrast, other researchers suggest that cell death occurs through necrosis rather than apoptosis because SR-A inhibits apoptosis in macrophages.38 As the plaque grows, the necrotic core grows as well, enhancing the plaque shoulder, where foam cells release proteases to facilitate plaque destruction. Patients suffering from late-stage atherosclerosis have more macrophage-rich areas in the arterial wall, implying that macrophages may be indicative of unstable plaques.39
Fibrous Plaques
The fibrous plaque is composed of a “necrotic core” containing lipids and the dead cell debris, with a fibrous cap that covers the core.1–3 The fibrous cap is constituted of smooth muscle cells (SMCs), a growing mass of extracellular lipid, and the accumulation of the SMC-derived ECM.3 Immune cells including macrophages, T cells and mast cells are found in the fibrous plaque.25 The cytokines and growth factors secreted by these immune cells are the key players in the plaque inflammation and vascular function.39–41 Other than this, it is also said that the migration and proliferation of SMCs are stimulated by a number of factors, including elevated levels of homocysteine and angiotensin-α.1 Homocysteine is able to damage the ECs, stimulating the proliferation of the SMCs,42 and angiotensin-α directly simulates the growth of the SMCs to prompt their secretion of ECM proteins. The fibrous cap is covered by a monolayer of endothelial cells, which sequesters peripheral blood from the subendothelial lesion before it ruptures. Death of the ECs, which is a process in the plaque rupture, may result from the cytokines produced by T cells, mast cells, a few B cells or probably even nature killer T cells.43
It is now clear that the ligation of CD40 and CD40L (also known as CD154) can also trigger the production of the pro-inflammatory cytokines and the proteases that degrade the matrix.44 Studies have shown that CD40-null mice have smaller lesions and fewer inflammations.45 INFγ, secreted by T cells, can inhibit EC and SMC growth and collagen synthesis, thereby weakening the plaque.46 Meanwhile, the collagenases and proteases produced by macrophages will destroy the ECM of the SMCs, and trigger plaque rupture.5 Interestingly, it has been shown that mice lacking the INFγ receptor have a reduction in atherosclerosis.47 Plaque growth and/or rupture can be inhibited by anti-inflammatory cytokines such as IL-10 and transforming growth factor β (TGFβ); these cytokines trigger collagen production and thus help to increase the stability of the fibrous cap.48
Advanced Lesion and Thrombosis
The degradation of matrix within the fibrous cap by various proteases such as collagenases, gelatinases and stromolysin will make the plaque more vulnerable and more susceptible to rupture.5 Tissue factor in the lesion lipid core is the key player in thrombosis initiation as well. It is produced by activated ECs and macrophages, enhanced by oxLDL or ligation of CD40 on CD40L.48 When the plaque ruptures, the fibrin meshwork (a tissue factor produced by the activated ECs) in the necrotic core is exposed to platelets in the blood, causing platelet adherence, that together with the necrotic core can lead to thrombosis.50 Ligation of CD40 expressed by macrophages strongly induces the expression of tissue factor.50 The maintenance of the fibrous plaque depends greatly on the balance of production and degradation of the matrix.1 As discussed earlier, INFγ produced by T cells will inhibit the generation of matrix proteins by the SMCs, while macrophages produce proteases to degrade the matrix. It is found that rupture often occurs in lesion edges, where foam cells are found in large numbers, so it is concluded that inflammatory factors may also play a role in thrombosis.3
Calcification and neovascularization are the two common features of advanced thrombosis.51 Calcification can be regarded as a process, where the matrix scaffold secreted by cells is calcified. Moultan et al. have shown that small vessel growth may be the conduit for the entry of inflammatory cells.52 Other molecules such as plasminogen activator may also be important in mediating thrombosis, as this factor may lead to the degradation of the fibrin meshwork.3
Conclusions
The new understanding of inflammation as a pivotal link in atherosclerosis opens various opportunities for therapy in this disease. Effective drugs for lowering the level of cholesterol have been developed.53 The use of statins has shown striking clinical benefits in lowering atherogenic lipoproteins, as well as preventing atherosclerotic complications.54 Clinical trials have validated that 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (drugs of the statin family) is helpful in preventing atherosclerotic complications.55 Recent experimental data have shown that some of the the benefit of statins may be due to its anti-inflammatory effect, unrelated to cholesterol reduction.55 Moreover, is has recently been established that another group of anti-atherosclerotic drugs, known as PPARs, can inhibit T cell activation in vitro. This is represented by decreased production of INFγ, TNFα and IL-2.56
These examples provide an illustration of the anti-inflammatory effects of existing therapies. Understanding the inflammatory pathways, in atherosclerosis, opens new possibilities for future treatments that could directly target the effectors of inflammation to implement novel treatments.
Interestingly, a level of complexity exists as nonspecific anti-inflammatory drugs, such as nonsteroidal anti-inflammatory drugs (NSAIDs) have been shown to be pro-atherosclerotic.57 It is suggested that NSAIDs increase the chances of thrombosis by affecting plasma lipoproteins, as well as promoting insulin resistance and sodium retention and inhibiting collagen and elastin formation.57 Therefore, NSAIDs may not be therapeutic alternatives in the chronic phases of atherosclerosis.
However, immunomodulation is still a possible therapeutic solution, although broad immunosuppressants would produce undesirable outcomes,53 therefore, we should try to develop more specific immunomodulators that would act on the key mechanisms of the atherogenic process. Potential targets are proinflammatory lipids such as lipoprotein-derived antigens, and signaling pathways leading to inflammatory responses.58
Finally, therapies that would target the inflammatory pathways triggered during atherosclerosis would undoubtedly have potential beneficial effect in tackling this disease.
Acknowledgements
This work was supported by a research grants from the NMRC/0817/2003. We thank A.-K. Fraser-Andrews for proofreading the manuscript.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/5321
References
- 1.Peter L. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.Tamminen M, Mottino G, Qiao H, Breslo JL, Frank JS. Ultrastructure of early lipid accumulation in apoE-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:847–853. doi: 10.1161/01.atv.19.4.847. [DOI] [PubMed] [Google Scholar]
- 3.Aldons JL. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 5.Libby P. Changing concepts of atherosclerosis. J Intern Med. 1999;247:349–358. doi: 10.1046/j.1365-2796.2000.00654.x. [DOI] [PubMed] [Google Scholar]
- 6.Gimbrone MA. Vascular endothelium, hemodynamic forces, and atherogenesis. Am J Pathol. 1999;155:1–5. doi: 10.1016/S0002-9440(10)65090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borén J, Olin K, Lee I, Chait A, Wight TN, Innerarity TL. Identification of the principal proteoglycan-binding site in LDL: A single-point mutation in apo-B100 severely affects proteoglycan interaction without affecting LDL receptor binding. J Clin Invest. 1998;101:2658–2664. doi: 10.1172/JCI2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein JL, Brown MS. Binding sites on macrophages that mediate uptake and degradation of aceylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci USA. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varki A. Selectin ligands. Proc Natl Acad Sci USA. 1994;91:7390–7397. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colling RG. P-selectin or intercellular adhesion molecule (ICAM1) deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J Exp Med. 2000;191:189–194. doi: 10.1084/jem.191.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cybulsky M, Gimbrone MA. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherosclerosis. Science. 1991;251:788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 12.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K, Takeya M, Sakashita N. Multifunctional roles of macrophages in the development and progression of atherosclerosis in humans and experimental animals. Med Elect Micros. 2002;35:179–203. doi: 10.1007/s007950200023. [DOI] [PubMed] [Google Scholar]
- 14.Shih PT. Blocking very late antigen-4 integrin decreases leukocyte entry and fatty streak formation in mice fed an atherogenic diet. Circ Res. 1999;84:345–351. doi: 10.1161/01.res.84.3.345. [DOI] [PubMed] [Google Scholar]
- 15.Sheikine Y, Hansson GK. Chemokines and atherosclerosis. Ann Intl Med. 2004;36:98–118. doi: 10.1080/07853890310019961. [DOI] [PubMed] [Google Scholar]
- 16.Quehenberger O. Molecular mechanisms regulating monocyte recruitment in atherosclerosis. J Lipid Research. 2005;46:1582–1590. doi: 10.1194/jlr.R500008-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2-/- mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 18.Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 19.Daugherty A, Webb NR, Rateri DL, King VL. Thematic review series: The immune system and atherogenesis. Cytokine regulation of macrophage functions in atherosclerosis. J Lipid Research. 2005;46:1812–1822. doi: 10.1194/jlr.R500009-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Zernecke A, Weber C. Inflammatory mediators in atherosclerotic vascular disease. Basic Res in Cardiol. 2005;100:93–101. doi: 10.1007/s00395-005-0511-6. [DOI] [PubMed] [Google Scholar]
- 21.Ostermann G, Weber KS, Zernecke A, Schröder A, Weber C. JAM-1 is a ligand of the beta2 integrin LFA-1 involved in transendothelial migration of leukocytes. Nature Immunology. 2002;3:151–158. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- 22.Rosenfeld ME, Ylä-Herttuala S, Lipton BA, Ord VA, Witztum JL, Steinberg D. Macrophage colony-stimulating factor mRNA and protein in atherosclerotic lesions of rabbits and humans. Am J Pathol. 1992;140:291–300. [PMC free article] [PubMed] [Google Scholar]
- 23.Yuri VB. Monocyte recruitment and foam cell formation in atherosclerosis. Micron. 2006;37:208–222. doi: 10.1016/j.micron.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci USA. 1995;92:8264–8268. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goran KH, Peter L. The immune response in atherosclerosis: A double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 26.Miller YI, Viriyakosol S, Binder CJ, Feramisco JR, Kirkland TN, Witztum JL. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2 and inhibits phagocytosis of apoptotic cells. J Biol Chem. 2003;278:1561–1568. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
- 27.Bobryshev YV. Dendritic cells and their control involvement in atherosclerosis. Curr Op Lipidol. 2000;11:511–517. doi: 10.1097/00041433-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–483. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- 29.Angeli V, Llodrá J, Rong JX, Satoh K, Ishii S, Shimizu T, Fisher EA, Randolph GJ. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity. 2004;21:561–574. doi: 10.1016/j.immuni.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Itabe H. Oxidized low density lipoproteins: What is understood and what remains to be clarified. Biol Pharm Bulletin. 2003;26:1–9. doi: 10.1248/bpb.26.1. [DOI] [PubMed] [Google Scholar]
- 31.Williams KJ, Tabas I. Lipoprotein retention-and clues for atheroma regression. Arterioscler Thromb Vasc Biol. 2005;25:1536–1540. doi: 10.1161/01.ATV.0000174795.62387.d3. [DOI] [PubMed] [Google Scholar]
- 32.Ivandic B, Castellani LW, Wang XP, Qiao JH, Mehrabian M, Navab M, Fogelman AM, Grass DS, Swanson ME, de Beer MC, de Beer F, Lusis AJ. Role of group II secretory phospholipase A2 in atherosclerosis: 1. Increased atherogenesis and altered lipoproteins in transgenic mice expressing group IIa phospholipase A2. Arterioscler Thromb Vasc Biol. 1999;19:1284–1290. doi: 10.1161/01.atv.19.5.1284. [DOI] [PubMed] [Google Scholar]
- 33.Marathe S, Kuriakose G, Williams KJ, Tabas I. Sphingomyelinase, an enzyme implicated in atherogenesis, is present in atherosclerotic lesions and binds to specific components of the subendothelial extracellular matrix. Arterioscler Thromb Vasc Biol. 1999;19:2648–2658. doi: 10.1161/01.atv.19.11.2648. [DOI] [PubMed] [Google Scholar]
- 34.Duval C, Chinetti G, Trottein F, Fruchart JC, Staels B. The role of PPARs in atherosclerosis. Trends Mol Med. 2002;8:422–430. doi: 10.1016/s1471-4914(02)02385-7. [DOI] [PubMed] [Google Scholar]
- 35.Accad M, Smith SJ, Newland DL, Sanan DA, King LE, Jr, Linton MF, Fazio S, Farese RV., Jr Massive xanthomatosis and altered composition of atherosclerotic lesions in hyperlipidemic mice lacking acyl CoA: Cholesterol acyltransferase 1. J Clin Invest. 2000;105:711–719. doi: 10.1172/JCI9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeichi M. Cadherin cell adhesion receptors as a morphogenic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- 37.Hegyi L, Hardwick SJ, Siow RC, Skepper JN. Macrophage death and the role of apoptosis in human atherosclerosis. J Hematother Stem Cell Res. 2001;10:27–42. doi: 10.1089/152581601750098192. [DOI] [PubMed] [Google Scholar]
- 38.Heidenreich S. Monocytes CD14: A multifunctional receptor engaged in apoptosis from both sides. J Leuk Biol. 1999;65:737–743. doi: 10.1002/jlb.65.6.737. [DOI] [PubMed] [Google Scholar]
- 39.Moreno, et al. Macrophage infiltration in acute coronary syndromes: Implications for plaque rupture. Circ. 1994;90:775–778. doi: 10.1161/01.cir.90.2.775. [DOI] [PubMed] [Google Scholar]
- 40.Weber C. Platelets and chemokines in atherosclerosis, partners in crime. Circ Res. 2005;96:612–616. doi: 10.1161/01.RES.0000160077.17427.57. [DOI] [PubMed] [Google Scholar]
- 41.Charo FI, Taubman BM. Chemokines in the pathogenesis of vascular diseases. Circ Res. 2004;95:858–866. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- 42.Gerhard GT, Duell PB. Homocysteine and atherosclerosis. Curr Opin Lipidol. 1999;10:417–429. doi: 10.1097/00041433-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Bobryshev YV, Lord RS. A S-100 positive cells in human arterial intima and in atherosclerotic lesions. Cardiovas Res. 1995;29:689–696. [PubMed] [Google Scholar]
- 44.Mach F, Schönbeck U, Sukhova GK, Atkinson E, Libby P. Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature. 1998;394:200–203. doi: 10.1038/28204. [DOI] [PubMed] [Google Scholar]
- 45.Fyfe AI, Qiao JH, Lusis AJ. Immune deficient mice develop typical atherosclerotic fatty streaks when fed an atherogenic diet. J Clin Invest. 1994;94:2516–2520. doi: 10.1172/JCI117622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friesel R, Komoriya A, Maciag T. Inhibition of endothelial cell proliferation by INF-γ. J Cell Biol. 1987;104:689–696. doi: 10.1083/jcb.104.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansson GK, Hellstrand M, Rymo L, Rubbia L, Gabbiani G. Interferon γ inhibits both proliferation and expression of differentiation-specific α-smooth muscle actin in arterial smooth muscle cells. J Exp Med. 1989;170:1595–1608. doi: 10.1084/jem.170.5.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 49.Schonbeck U, Mach F, Sukhova GK, Herman M, Graber P, Kehry MR, Libby P. CD40 ligation induces tissue factor expression in human vascular smooth muscle cells. Am J Pathol. 2000;156:7–14. doi: 10.1016/S0002-9440(10)64699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mach F, Schönbeck U, Bonnefoy JY, Pober JS, Libby P. Activation of monocyte/macrophage functions related to acute atheroma complication by ligation of CD40: Induction of collagenases, stromelysin, and tissue factor. Circ. 1997;96:396–399. doi: 10.1161/01.cir.96.2.396. [DOI] [PubMed] [Google Scholar]
- 51.Watson KE, Boström K, Ravindranath R, Lam T, Norton B, Demer LL. TGF-beta 1 and 25-hydroxycholesterol stimulate osteoblast-like vascular cells to calcify. J Clin Invest. 1994;93:2106–2113. doi: 10.1172/JCI117205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moultan KS, Folkman J. In: Molecular Basis of Cardiovascular Disease. Chien KR, editor. Philadelphia: Saunders; 1999. pp. 393–410. [Google Scholar]
- 53.Hansson GK. Immune Mechanisms in atherosclerosis. Arterioscles Thromb Vasc Biol. 2001;21:1876–1890. doi: 10.1161/hq1201.100220. [DOI] [PubMed] [Google Scholar]
- 54.Assmann G, Cullen P, Jossa F, Lewis B, Mancini M. Coronary heart disease: Reducing the risk. The scientific background to primary and secondary prevention of coronary heart disease: A worldwide view. International Task force for the Prevention of Coronary Heart disease. Arterioscler Thromb Vasc Biol. 1999;19:1819–1824. doi: 10.1161/01.atv.19.8.1819. [DOI] [PubMed] [Google Scholar]
- 55.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 56.Marx N, Kehrle B, Kohlhammer K, Grüb M, Koenig W, Hombach V, Libby P, Plutzky J. PPAR activators as anti-inflammatory mediators in human T lymphocytes: Implications for atherosclerosis and transplantation-associated arteriosclerosis. Circ Res. 2002;90:703–710. doi: 10.1161/01.res.0000014225.20727.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fitzgerald GA. Coxibs and cardiovascular disease. N Engl J Med. 2004;351:1709–1711. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- 58.Hasson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:1876–1890. doi: 10.1161/hq1201.100220. [DOI] [PubMed] [Google Scholar]