Abstract
The neurofibromatosis-2 (NF2) tumor suppressor protein, merlin or schwannomin, inhibits cell proliferation by modulating the growth activities of its binding partners, including the cell surface glycoprotein CD44, membrane-cytoskeleton linker protein ezrin and PIKE (PI 3-kinase enhancer) GTPase, etc. Merlin exerts its growth suppressive activity through a folded conformation that is tightly controlled through phosphorylation by numerous protein kinases including PAK, PKA and Akt. Merlin inhibits PI 3-kinase activity through binding to PIKE-L. Now, we show that merlin is a physiological substrate of Akt, which phosphorylates merlin on both T230 and S315 residues. This phosphorylation abolishes the folded conformation of merlin and inhibits its association with PIKE-L, provoking merlin polyubiquitination and proteasome-mediated degradation. This finding demonstrates a negative feed-back loop from merlin/PIKE-L/PI 3-kinase to Akt in tumors. The proliferation repressive activity of merlin is also partially regulated by S518 phosphorylation. Thus, Akt-mediated merlin T230/S315 phosphorylation, combined with S518 phosphorylation by PAK and PKA, provides new insight into abrogating merlin function in the absence of merlin mutational inactivation.
Key Words: Akt, merlin, neurofibromatosis, phosphorylation, cell invasion and migration
Neurofibromatosis 2 (NF2) is a dominantly inherited disorder characterized by bilateral occurrence of vestibular schwannomas and other brain tumors, including meningiomas and ependymomas.1 The NF2 tumor suppressor protein merlin belongs to the band 4.1 family of cytoskeleton-associated proteins.2,3 Merlin isoform I possesses a “closed” conformation via an NTD (N-terminal domain)/CTD (Carboxy terminal domain) intramolecular interaction. In contrast, the alternatively spliced merlin isoform II exists in an “open” conformation that cannot function as a negative growth regulator.4 Merlin with NF2 patient missense mutations in the NTD or CTD exhibit an “open” conformation and do not suppress cell growth.5 Merlin plays a key role in regulating cell proliferation and cell migration. Merlin exerts its growth suppressive activity through intramolecular folding that dictates its binding affinities to various cellular partners including HRS (hepatocyte growth factor regulated tyrosine kinase substrate), CD44 cell surface glycoprotein, schwannomin interacting protein-1 (SCHIP1), βII-spectrin or fodrin, PIKE-L GTPase and other ERM proteins.6–10 For instance, CD44 preferentially associates with hypophosphorylated merlin, and relatively little phosphorylated merlin binds CD44. Interference with merlin binding to CD44 impairs merlin growth suppression in RT4 rat schwannoma cells.11
We have previously shown that the PIKE/PI 3-kinase signaling pathway is negatively regulated by protein 4.1N, a neuronal selective isoform of band 4.1 superfamily.12 Recently, we show that PIKE-L is an important mediator of merlin growth suppression. We show that merlin blocks cell proliferation by inhibiting PI 3-kinase through binding to PIKE-L. Interestingly, wild-type merlin, but not patient-derived mutant (L64P), binds PIKE-L and inhibits PI 3-kinase activity. This suppression of PI 3-kinase activity results from merlin disrupting the binding of PIKE-L to PI 3-kinase. Mutation of PIKE-L with Proline 187 into Leucine disrupts its interaction with merlin. Accordingly, merlin suppression of PI 3-kinase activity as well as schwannoma cell growth is abrogated by a single PIKE-L point mutation (P187L).10
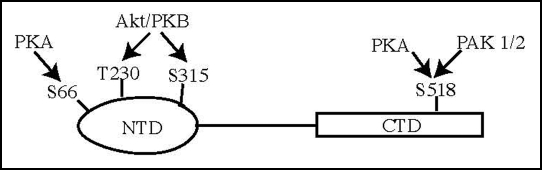
Merlin is phosphorylated on S518 by members of the PAK family of kinases, including PAK1 and PAK2,13–15 which mislocates merlin from the plasma membrane to the cytoplasm. A merlin mutant that mimics S518 phosphorylation (S518D) cannot suppress cell growth or motility in RT4 rat schwannoma cells, and leads to dramatic changes in cell morphology and actin cytoskeleton organization.16 S518 phosphorylation results in impaired merlin NTD/CTD folding as well as altered interactions with critical merlin associated proteins, including CD44 and HRS.17 Recently, Alfthan and colleagues demonstrated that Protein Kinase-A (PKA) induces merlin phosphorylation on both N-and C-terminal residues.18 In addition to S518 phosphorylation, PKA can phosphorylate merlin at S66 in the N-terminal domain (Fig. 1). When PAK activity is suppressed, merlin can still be phosphorylated by PKA in cells, indicating that these two kinases function independently. The N-terminus of ezrin strongly binds to a PKA-phosphorylated, but not unphosphorylated, merlin CTD. In contrast, PAK2-induced S518 phosphorylation has a minimal effect on the interaction between full-length merlin and full-length ezrin.17 Besides regulation of cell growth, merlin also mediates cell motility presumably through directly binding to actin cytoskeleton.19 Depletion of merlin in normal fibroblast results in enhanced cell invasion. Nevertheless, expression of merlin attenuates Y397 phosphorylation on FAK, an essential player in cell migration and invasion. This observation might provide a molecular mechanism accounting for merlin inhibitory activity in cell motility.20
Figure 1.
Merlin phosphorylation sites by various kinases.
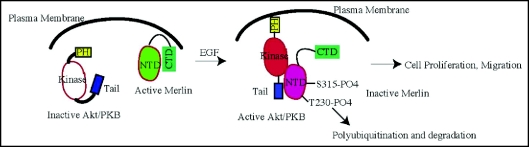
In addition to PAK and PKA, we show that Akt potently phosphorylates merlin at both T230 and S315 residues. Blocking one site phosphorylation abolishes the other site phosphorylation by Akt, indicating that these two phosphorylation sites are mutually regulated.21 The physiological significance of the tight control on merlin phosphorylation by Akt remains incompletely understood. Presumably, only when mitogenic signal or oncogenic stress is strongly enough to provoke cell proliferation or migration, does Akt simultaneously phosphorylate both sites. Akt phosphorylation of merlin attenuates the NTD/CTD interaction and inhibits its binding activity to PIKE-L, CD44 and ezrin. Further, phosphorylation mediates the biological activities of merlin, as expression of a phosphomimetic merlin mutant (T230DS315D) increases cell motility and proliferation in a rat schwannoma cell line (Fig. 2). By contrast, expression of a mutant (T230A/S315A) that was unable to undergo phosphorylation inhibited cell growth and motility. The F1 motif in FERM proteins including merlin exhibits an ubiquitin-like structure. This domain facilitates MDM2 degradation and stimulates the ubiquitination and degradation of TRBP, a double-stranded RNA binding protein. Surprisingly, inhibition of the proteasome does not affect total merlin protein levels in human glioblastoma cells, but leads to a marked increase of phospho-S315 merlin. Simultaneous treatment with MG132, which blocks proteasome-mediated degradation and PI 3-kinase inhibitor, wortmannin, which inhibits Akt phosphorylation of merlin, substantially enhances merlin levels. Coimmunoprecipitation studies demonstrate that Akt-phosphorylated merlin is rapidly ubiquitinated, presumably by spectrin, which binds to merlin and possesses ubiquitin-conjugating and ubiquitin E3 ligase function. However, S518 phosphorylation fails to trigger merlin ubiquitination, suggesting that Akt, but not PAK or PKA, phosphorylation selectively elicits merlin ubiquitination. Using a panel of human primary nervous system tumors, we found that merlin phosphorylation by Akt also mediates its degradation in primary tumors. Accordingly, tumors that possess high levels of phospho-Akt exhibited low levels of merlin. Therefore, our data suggest a novel role for Akt in promoting phosphorylation and subsequent degradation of merlin. Loss of merlin has been linked to schwannomas and other nervous system tumors, and these results indicate that inhibitors for PI 3-kinase/Akt pathway might restore merlin function in tumors.
Figure 2.
The model for Akt interaction with merlin and its phosphorylation.
Acknowledgements
This work is supported by a Research-Initiated Award from Department of Defense (NF05009, W81XWH0610286) and RO1 (CA117872) from NIH.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/5192
References
- 1.Eldridge R. Central neurofibromatosis with bilateral acoustic neuroma. Adv Neurol. 1981;29:57–65. [PubMed] [Google Scholar]
- 2.Rouleau GA, Merel P, Lutchman M, Sanson M, Zucman J, Marineau C, Hoang-Xuan K, Demczuk S, Desmaze C, Plougastel B, et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993;363:515–521. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- 3.Trofatter JA, MacCollin MM, Rutter JL, Murrell JR, Duyao MP, Parry DM, Eldridge R, Kley N, Menon AG, Pulaski K, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;72:791–800. doi: 10.1016/0092-8674(93)90406-g. [DOI] [PubMed] [Google Scholar]
- 4.Sherman L, Xu HM, Geist RT, Saporito-Irwin S, Howells N, Ponta H, Herrlich P, Gutmann DH. Interdomain binding mediates tumor growth suppression by the NF2 gene product. Oncogene. 1997;15:2505–2509. doi: 10.1038/sj.onc.1201418. [DOI] [PubMed] [Google Scholar]
- 5.Gutmann DH, Geist RT, Xu H, Kim JS, Saporito-Irwin S. Defects in neurofibromatosis 2 protein function can arise at multiple levels. Hum Mol Genet. 1998;7:335–345. doi: 10.1093/hmg/7.3.335. [DOI] [PubMed] [Google Scholar]
- 6.Scoles DR, Huynh DP, Morcos PA, Coulsell ER, Robinson NG, Tamanoi F, Pulst SM. Neurofibromatosis 2 tumour suppressor schwannomin interacts with betaII- spectrin. Nat Genet. 1998;18:354–359. doi: 10.1038/ng0498-354. [DOI] [PubMed] [Google Scholar]
- 7.Meng JJ, Lowrie DJ, Sun H, Dorsey E, Pelton PD, Bashour AM, Groden J, Ratner N, Ip W. Interaction between two isoforms of the NF2 tumor suppressor protein, merlin, and between merlin and ezrin, suggests modulation of ERM proteins by merlin. J Neurosci Res. 2000;62:491–502. doi: 10.1002/1097-4547(20001115)62:4<491::AID-JNR3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 8.Goutebroze L, Brault E, Muchardt C, Camonis J, Thomas G. Cloning and characterization of SCHIP-1, a novel protein interacting specifically with spliced isoforms and naturally occurring mutant NF2 proteins. Mol Cell Biol. 2000;20:1699–1712. doi: 10.1128/mcb.20.5.1699-1712.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun CX, Haipek C, Scoles DR, Pulst SM, Giovannini M, Komada M, Gutmann DH. Functional analysis of the relationship between the neurofibromatosis 2 tumor suppressor and its binding partner, hepatocyte growth factor-regulated tyrosine kinase substrate. Hum Mol Genet. 2002;11:3167–3178. doi: 10.1093/hmg/11.25.3167. [DOI] [PubMed] [Google Scholar]
- 10.Rong R, Tang X, Gutmann DH, Ye K. Neurofibromatosis 2 (NF2) tumor suppressor merlin inhibits phosphatidylinositol 3-kinase through binding to PIKE-L. Proc Natl Acad Sci USA. 2004;101:18200–18205. doi: 10.1073/pnas.0405971102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison H, Sherman LS, Leff J, Banine F, Isacke C, Haipek CA, Gutmann DH, Ponta H, Herrlick P. The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev. 2001;15:968–980. doi: 10.1101/gad.189601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye K, Hurt KJ, Wu FY, Fang M, Luo HR, Hong JJ, Blackshaw S, Ferris CD, Snyder SH. Pike: A nuclear gtpase that enhances PI3kinase activity and is regulated by protein 4.1N. Cell. 2000;103:919–930. doi: 10.1016/s0092-8674(00)00195-1. [DOI] [PubMed] [Google Scholar]
- 13.Kissil JL, Johnson KC, Eckman MS, Jacks T. Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on merlin localization. J Biol Chem. 2002;277:10394–10399. doi: 10.1074/jbc.M200083200. [DOI] [PubMed] [Google Scholar]
- 14.Xiao GH, Beeser A, Chernoff J, Testa JR. p21-activated kinase links Rac/Cdc42 signaling to merlin. J Biol Chem. 2002;277:883–886. doi: 10.1074/jbc.C100553200. [DOI] [PubMed] [Google Scholar]
- 15.Shaw RJ, Paez JG, Curto M, Yaktine A, Pruitt WM, Saotome I, O'Bryan JP, Gupta V, Ratner N, Der CJ, et al. The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev Cell. 2001;1:63–72. doi: 10.1016/s1534-5807(01)00009-0. [DOI] [PubMed] [Google Scholar]
- 16.Surace EI, Haipek CA, Gutmann DH. Effect of merlin phosphorylation on neurofibromatosis 2 (NF2) gene function. Oncogene. 2004;23:580–587. doi: 10.1038/sj.onc.1207142. [DOI] [PubMed] [Google Scholar]
- 17.Rong R, Surace EI, Haipek CA, Gutmann DH, Ye K. Serine 518 phosphorylation modulates merlin intramolecular association and binding to critical effectors important for NF2 growth suppression. Oncogene. 2004;23:8447–8454. doi: 10.1038/sj.onc.1207794. [DOI] [PubMed] [Google Scholar]
- 18.Alfthan K, Heiska L, Gronholm M, Renkema GH, Carpen O. Cyclic AMP-dependent protein kinase phosphorylates Merlin at serine 518 independently of P21-activated kinase and promotes Merlin-Ezrin heterodimerization. J Biol Chem. 2004;279:18559–18566. doi: 10.1074/jbc.M313916200. [DOI] [PubMed] [Google Scholar]
- 19.Xu HM, Gutmann DH. Merlin differentially associates with the microtubule and actin cytoskeleton. J Neurosci Res. 1998;51:403–415. doi: 10.1002/(SICI)1097-4547(19980201)51:3<403::AID-JNR13>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Poulikakos PI, Xiao GH, Gallagher R, Jablonski S, Jhanwar SC, Testa JR. Re-expression of the tumor suppressor NF2/merlin inhibits invasiveness in mesothelioma cells and negatively regulates FAK. Oncogene. 2006;25:5960–5968. doi: 10.1038/sj.onc.1209587. [DOI] [PubMed] [Google Scholar]
- 21.Tang X, Jang SW, Wang X, Liu Z, Bahr SM, Sun SY, Brat D, Gutmann DH, Ye K. Akt phosphorylation regulates the tumour-suppressor merlin through ubiquitination and degradation. Nat Cell Biol. 2007;9:1199–1207. doi: 10.1038/ncb1641. [DOI] [PubMed] [Google Scholar]