Abstract
Ozone is the main photochemical oxidant that causes leaf damage in many plant species, and can thereby significantly decrease the productivity of crops and forests. When ozone is incorporated into plants, it produces reactive oxygen species (ROS), such as superoxide radicals and hydrogen peroxide. These ROS induce the synthesis of several plant hormones, such as ethylene, salicylic acid, and jasmonic acid. These phytohormones are required for plant growth, development, and defense responses, and regulate the extent of leaf injury in ozone-fumigated plants. Recently, responses to ozone have been studied using genetically modified plants and mutants with altered hormone levels or signaling pathways. These researches have clarified the roles of phytohormones and the complexity of their signaling pathways. The present paper reviews the biosynthesis of the phytohormones ethylene, salicylic acid, and jasmonic acid, their roles in plant responses to ozone, and multiple interactions between these phytohormones in ozone-exposed plants.
Key words: cross-talk, ethylene, jasmonic acid, ozone, phytohormones, programmed cell death, salicylic acid, signaling pathways
Introduction
As the Earth's population and industrial productivity increase, air pollution is becoming one of the most serious environmental problems. Tropospheric concentrations of ozone, which is a major photochemical oxidant, have increased markedly since the turn of the last century,1 causing extensive damage to natural and cultivated plants.2 Ambient ozone concentrations in Europe, Japan and the United States usually vary between 20 and 60 ppb during seasons with high light intensity, but acute ozone peaks exceeding 200 ppb are occasionally observed around large cities.
Exposure to ozone can result in foliar lesions such as chlorosis and necrosis, but the appearance and severity of this leaf damage differs among plant species and varieties. For example, exposure of two tobacco (Nicotiana tabacum) cultivars, ‘Bel-B’ and ‘Bel-W3’, to acute ozone levels produced marked leaf damage only in ‘Bel-W3’.3,4 Natural genetic variation in ozone tolerance has also been reported in Arabidopsis accessions between the ozone-tolerant Col-0 ecotype and the ozone-sensitive accession Ws-2.5
After entering the leaf through stomata, ozone dissolves in the apoplast, where it generates superoxide (O2-) and hydrogen peroxide (H2O2), which then lead to the production of additional reactive oxygen species (ROS) in oxidative bursts, most probably through the action of apoplastic NADPH oxidase.6 To remove and detoxify excess ROS, plants have evolved both enzymatic and nonenzymatic antioxidant defenses such as the production of ascorbic acid, glutathione, α-tocopherol, and catalases.7 Of these, glutathione is regarded as a central component of the antioxidant defense in higher plants.8 Glutathione has a higher affinity for oxidants than other antioxidant molecules, such as ascorbic acid.9
Acute ozone exposure results in the activation of a programmed cell death (PCD) response that resembles the hypersensitive response observed in plant-pathogen interactions.10–12 Ozone-induced PCD is characterized by an oxidative burst and by the coincident induction of pathogenesis-related (PR) proteins and antioxidant defense genes such as GST1 and various ascorbate peroxidase genes.5,13 The participation of several phytohormones in response to acute ozone exposure, downstream of ROS production, has been well established. Indeed, exposure to acute levels of ozone activates signal-transduction pathways for phytohormones such as ethylene, salicylic acid (SA), and jasmonic acid (JA), leading to downstream responses such as antimicrobial defenses.14,15 Recent studies have demonstrated that these signaling pathways do not act independently, and that the degree of lesion formation induced by ozone is influenced by cross-talk between these signaling pathways.13,16–24 In the present paper, I review the role of phytohormones and their interactions in the processes responsible for ozone-induced cell death.
Early Responses in Ozone-Exposed Plants
Plants have evolved systems capable of sensing and responding to environmental changes, including increases in ozone levels. It has been clearly established that ozone is a highly toxic molecule responsible for the generation of foliar lesions, but the initial site of ozone reactions in plants is not yet completely understood. Based on measurements of ozone flux into leaves, it has been suggested that ozone does not penetrate deeply into intercellular spaces, but rather degrades the cell wall and plasma membrane.25 This raises the question of how ozone generates physiological responses in plants. The plant cell wall contains many phenolic compounds and the plasma membrane contains a high proportion of unsaturated lipids. It is thus likely that the first set of molecules that will react with ozone will be encountered within the cell wall regions just outside the plasma membrane and that highly toxic ROS will form in the tissues.26
These ozone-derived ROS could trigger a wide array of signal cascades, such as alterations in the physical and chemical properties of the plasma membrane, changes in calcium influx, and the induction of protein kinases. Thus, the initial recognition of ozone by the plant might result from the response of cell walls and plasma membranes to the generation of ROS. After this sensing step has occurred, signals generated at the receptors or sensors would be converted into cellular responses by means of various signal-transduction pathways. The earliest signaling events, such as protein phosphorylation or dephosphorylation and calcium influx, can occur within a few minutes. These changes are followed by the production of several signaling compounds, including ROS, ethylene, SA, and JA.16,27–32 Changes in global gene expression in response to these primary and secondary signals eventually alter the metabolism and physiology of plants, and this leads to their response to the new environment that stimulated the change.
Mitogen-activated protein kinase (MAPK) cascades are major pathways downstream of sensors and receptors that transduce extracellular stimuli into intracellular responses in eukaryotes.33–37 Recent studies have demonstrated that WIPK and SIPK, two tobacco (Nicotiana tabacum) MAPKs, as well as their functional orthologs in other plant species, including MPK3 and MPK6 in Arabidopsis thaliana, are activated in plants under ozone stress.38,39 Ozone-induced activation of SIPK, MPK6 and MPK3 occurs within 10 to 30 minutes, representing one of the earliest responses in plants under stress, and potentially allows these MAPKs to influence a variety of other early, intermediate, and late stress responses such as the accumulation of phytohormones. In addition, constitutive MAPK activation has been shown to lead to hypersensitivity response (HR)-like cell death, suggesting that MAPK signaling is a part of the ROS-induced PCD pathway.40 This also indicates that MAPK activation might be needed for the early phases of cell death caused by ozone. However, whether the activation of AtMPK3 and AtMPK6 causes or results from the increased cell death is unclear; for example, no direct evidence has been obtained for the relationship between the magnitude of cell death in the Arabidopsis rcd1 mutant, with high ozone sensitivity,18 and activation of AtMPK6, AtMPK3, or both in this mutant.12 Furthermore, the ozone-sensitive jar1 Arabidopsis mutant has MAPK activity similar to that of the ozone-tolerant Col-0.39 Samuel and Ellis41 described the complicated functioning of SIPK in ozone tolerance. Ozone sensitivity in transgenic tobacco plants that show inhibition or constitutive activation of SIPK activity is higher than that in wild-type plants. As well as AtMPK3/WIPK and AtMPK6/SIPK, MPK4 also has an important role in the plant defense system against ozone. Transgenic tobacco with repressed expression of NtMPK4, a tobacco homolog of Arabidopsis MPK4 (AtMPK4), showed enhanced sensitivity to ozone.42 Conversely, transgenic tobacco plants with higher activity of NtMPK4 as a result of overproduction of SIPKK or the constitutively active SIPKKEE type showed enhanced resistance to ozone. NtMPK4's role in ozone tolerance is thought to be caused by its regulation of stomatal closure in an abscisic acid (ABA)-independent manner, because NtMPK4-silenced plants showed high stomatal conductance and reduced stomatal closure on exposure to ozone.
Another early event triggered by ozone exposure is elevation of the concentration of free cytosolic calcium ion. Calcium functions as a second messenger in a wide range of signal-transduction networks in plants, linking the perception of a range of stimuli and stresses to downstream cellular responses.43 Clayton et al.44 showed that when plants were exposed to acute levels of ozone, a short-lived spike-like elevation of free cytosolic calcium was observed within 5 min, followed by a second, more gradual, elevation of calcium 15 min after the ozone exposure. The second peak was smaller than the first one, but persisted for more than 30 min. The second of the two cytosolic calcium peaks was eliminated by pretreatment of the plants with lanthanum chloride, but the first peak was still detected. In the presence of lanthanum chloride, the induction of a gene for the antioxidant defense enzyme glutathione-S-transferase (GST) in response to ozone decreased, suggesting that the second peak is necessary to induce GST expression.44 The biphasic calcium response to ozone exposure has been observed in the aboveground portion of plants, and depended on the rate of increase in ozone level.45 Experiments utilizing inhibitors of antioxidant metabolism and mutants with impaired glutathione synthesis demonstrated that the level of the first calcium peak in the aboveground plant parts depended on the redox status of the plant, including the levels of compounds such as glutathione and ascorbic acid.45 In addition, Miles et al.46 have demonstrated in tobacco suspension-cultured cells that an influx of extracellular calcium ions plays an essential role in the activation of SIPK in response to treatment with ROS. This indicates that calcium influx caused by ozone might trigger part of the observed ROS-induced PCD through the activation of MAPKs. Furthermore, ozone-induced changes in levels of free cytosolic calcium may lead to phosphorylation of one of the subunits of NADPH-oxidase known to generate ROS.47 This fact suggests that calcium influx in response to ozone exposure could trigger NADPH oxidase-dependent ROS production. However, the detailed mechanisms by which ROS alter free calcium levels are not yet known. Recently, Kadono et al.3 showed that pretreatment of Ca2+ chelators inhibit ozone-induced cell death in tobacco (BelW3) suspension culture. Therefore, calcium signaling initiated by ozone-dependent Ca2+ influx possibly links the primary and secondary oxidative bursts that is achieved by activation of NADPH-oxidase.
Salicylic Acid Acts as a Main Molecule in the Induction of PCD by Ozone
SA is a major phenylpropanoid compound whose biosynthesis is triggered by various biotic and abiotic stresses. Accumulation of SA has also been observed in ozone-exposed plants.48 It had originally been thought that SA was synthesized only from phenylalanine via t-cinnamic acid and benzoic acid in tobacco, potato (Solanum tuberosum) and Arabidopsis.49–53 Phenylalanine ammonia-lyase (PAL), which catalyzes the transformation of phenylalanine into t-cinnamic acid, is a rate-limiting enzyme in the production of phenylpropanoid compounds in tobacco (Fig. 1).54,55 SA accumulation decreases in pathogen-challenged or elicitor-treated plants when endogenous PAL expression is suppressed by means of genetic manipulation or treatment with the PAL inhibitor 2-aminoindan-2-phosphonic acid.51,52,56 These results suggest that PAL is an important enzyme in the pathway of SA synthesis.
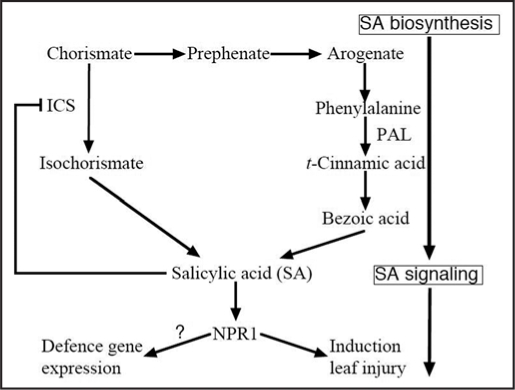
Figure 1.
Proposed pathways for salicylic acid biosynthesis and signaling in ozone-exposed plants. There are two pathways for salicylic acid (SA) biosynthesis in plants. Arabidopsis mutant sid2, that have a defect in ICS, does not accumulate salicylic acid (SA) in response to ozone, implying that SA is synthesized from isochorismate pathway (down from Chorismate) in Arabidopsis. However, another evidences support synthesis of SA from phenylalanine (PAL) pathway (right from Chorismate) in plants except for Arabidopsis. Final product (SA) of this pathway inhibits ICS expression and/or ICS activity. NPR; nonexpressor of PR-1.
However, Wildermuth et al.57 found another SA biosynthesis pathway that starts from chorismate and proceeds via isochorismate in pathogen-infected Arabidopsis (Fig. 1). This pathway has also been reported in Pseudomonas aeruginosa, and isochorismate synthase (ICS) is the rate-limiting enzyme for this pathway.58 Thus, two different SA synthesis pathways have been identified in plants. An unresolved issue is which pathway is used for SA synthesis when plants are exposed to ozone. In tobacco plants, 14C-labeled benzoic acid (a precursor of SA in the phenylalanine pathway) is metabolized to SA in ozone-exposed tobacco leaves, but the activity and mRNA level of ICS did not increase.24 In contrast, ICS activity increased in ozone-exposed Arabidopsis. Moreover, an Arabidopsis mutant, salicylic acid induction-deficient 2 (sid2),59 which completely lacks ICS1 activity showed low levels of SA accumulation during ozone exposure.24 These results suggest that SA is synthesized in response to ozone exposure via benzoic acid from phenylalanine in tobacco leaves but via isochorismate in Arabidopsis (Fig. 1). A gene encoding ICS (AJ006065) has been isolated and ICS has been purified from cell cultures of Catharanthus roseus.60 Putative ICS genes have also been found in rice (AP008215), tobacco (AY740529), tomato (DQ149918) and hot pepper (AY743431), but whether the isochorismate pathway is used for SA biosynthesis in these plants has not been reported. Therefore, Arabidopsis is currently the only plant in which SA is known to be synthesized via the isochorismate pathway.
Although it is unclear why different SA biosynthesis pathways are induced by ozone in different plant species, understanding the causes may provide interesting evidence for the evolution of species ranging from bacteria to plants because the ICS pathway is also used in SA biosynthesis in some bacteria. In P. aeruginosa, PchA, which is functionally identical to ICS, limits the production of SA, suggesting that ICS is the rate-limiting enzyme for SA biosynthesis in bacteria.58 In ozone-exposed Arabidopsis, SA accumulation accompanies an increase in ICS1 expression and ICS activity.61 Therefore, it is likely that ICS is the rate-limiting enzyme for SA biosynthesis in Arabidopsis too. A recent study showed that the ICS pathway in Arabidopsis seems to be negatively regulated by SA signaling, because the level of ICS1 expression and the activity of ICS during ozone exposure were both elevated significantly in two mutants (npr1 and eds5) that are deficient in SA signaling and in transgenic plants (NahG) that are deficient in SA accumulation.61 Moreover, SA treatments suppressed the enhancement of ICS1 expression by ozone.61 This result could support the presence of negative feedback regulation of SA biosynthesis by the final product, SA.
As described above, SA accumulates in ozone-exposed plants, and high levels of SA correlate with the formation of leaf lesions. Expression of NahG, a bacterial salicylate hydroxylase gene, in the tobacco cultivar ‘Xanthi’ resulted in an inability to accumulate SA62 and reduced lesion formation in response to ozone exposure.63 Similarly, the ozone-sensitive Arabidopsis ecotype Cvi-0 and the rcd1 mutant (which hyperaccumulates SA in response to ozone exposure) became markedly more tolerant of ozone when transformed with NahG or crossed with the npr1 mutant, which lacks SA signaling.12,64 Furthermore, when exogenous SA was added to superoxide-treated Col-0, it significantly enhanced the induction of cell death.65 These results indicate that ozone-induced production of SA acts as a signal that amplifies downstream signals that lead to cell death in a process similar to what occurs in pathogen-infected plants.
On the other hand, other studies have shown that SA induces a defense response in plants exposed to ozone. A NahG-transformed line of the ozone-tolerant Arabidopsis Col-0 ecotype showed remarkably greater ozone sensitivity than Col-0.64,66 As in the case of ethylene (discussed in more detail in the next section), ozone-inducible SA seems to have a dual function that depends on its production level. The ozone-sensitive Arabidopsis ecotype Cvi-0 accumulates more than three times as much SA as the ozone-tolerant Col-0 ecotype.64 In Col-0 plants, the induction of genes responsible for the production of antioxidants (e.g., cytosolic ascorbate peroxidase, chloroplastic glutathione reductase, chloroplastic glutathione peroxidase, and cytosolic GST) in response to ozone treatment were repressed in NahG-transformed plants.64 These results suggest that although optimal concentrations of SA are required to induce antioxidant defense responses and maintain a optimal cellular redox state, high levels of SA may activate the PCD pathway and cause ozone sensitivity.
Ethylene Promotes the Development of Leaf Injury in Response to Ozone
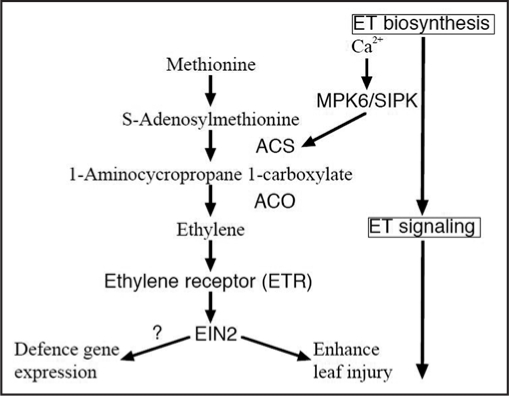
Ethylene is involved in regulating plant responses to both biotic and abiotic stresses, in addition to its functions in plant growth and development.67–69 Increased ethylene biosynthesis occurs in plants under a wide variety of stresses. The two key steps in ethylene biosynthesis are the conversion of S-adenosyl-L-methionine to 1-aminocyclopropane-1-carboxylic acid (ACC) and the oxidative cleavage of ACC to form ethylene (Fig. 2).67,70,71 The enzymes catalyzing these two reactions are ACC synthase (ACS) and ACC oxidase (ACO), respectively. Both enzymes are encoded by small gene families. In general, the basal activity level of ACS is very low in tissues that do not produce a significant amount of ethylene. Stress-induced ethylene production is associated with a rapid increase in cellular ACS activity. In contrast, ACO activity is constitutively present in most vegetative tissues. Therefore, ACS is the rate-limiting enzyme and governs the major regulatory step in stress-induced ethylene production.69–71 In ozone-exposed plants, LE-ACS1A, LE-ACS2, and LE-ACS6 are induced in tomato, and expression of ST-ACS4 and ST-ACS5 is increased in potato.72,73 The Arabidopsis genome encodes nine ACS genes, of which eight are functional (ACS2, ACS4-9, and ACS11) and one is nonfunctional (ACS1).74 Among these genes, only the expression of ACS6 is induced by ozone.75 This suggests that ACS6 is a key gene that triggers ethylene evolution in ozone-exposed Arabidopsis. Indeed, some studies have shown a correlation between the induction of ACS6 and an increase in ethylene levels.5,18 Recently, Liu and Zhang76 showed that phosphorylation of ACS2 and ACS6 by MPK6 leads to the accumulation of ACS (Fig. 2), and thus leads to elevated levels of cellular ACS activity, indicating that ozone-induced ethylene evolution might be regulated not only by the transcription level of ACS6, but also post-transcriptionally through the MAPK signaling pathway.
Figure 2.
Proposed pathways for ethylene biosynthesis and signaling in ozone-exposed plants. Activity of ACC synthase (ACS), the key enzyme of this pathway, is regulated with MPK6 in Arabidopsis or SIPK in tobacco, which activity is controlled with cytosolic free Ca2+. Ethylene binds to ethylene receptors (e.g., ETR), and ethylene signal is transmitted via EIN2.
Ozone-induced ethylene evolution was first identified by Craker,77 and Tingey et al.78 subsequently showed a correlation between the extent of leaf injury and the rate of ozone-induced ethylene production in various plant species. Ozone-sensitive ‘Bel-W3’ tobacco produces a higher level of ethylene than ozone-tolerant ‘Bel-B’ when these plants are exposed to ozone.79 Moreover, treatment with inhibitors for ethylene biosynthesis attenuates injury to plant leaves under ozone exposure.80 These results indicate that ethylene is involved in promotion of the development of ozone-induced leaf damage. Two hypotheses have been proposed for the mechanism of the damage-promoting effect of ozone-induced ethylene: the first assumes that the effect is due to the production of highly toxic substances such as free radicals and aldehydes through direct chemical reactions between ethylene and ozone,81,82 and the other proposes that ethylene serves as a signal molecule through its binding to one or more receptors.83 Some experiments using pretreatment with an inhibitor of ethylene reception were carried out to assess which hypothesis is correct. After treatment with inhibitors of ethylene reception, ozone-induced ethylene emission still occurred, but leaf damage caused by ozone decreased.5,18,83 This suggests that the ethylene signaling pathway must be activated for ozone to cause leaf damage.
Although ozone-inducible ethylene production clearly appears to promote the development of leaf injury, details of how ethylene functions in the development of leaf damage caused by ozone have not yet been clarified. Recently, Tanaka et al.84 showed that ethylene delays stomatal closure by inhibiting the ABA signaling pathway. ABA is involved in responses to several abiotic stresses and is a well-known regulator of stomatal closure. Thus, it is hypothesized that the delayed stomatal closure caused by ozone-induced ethylene production may result in a higher influx of ozone into leaves, and that this might trigger the promotion of the development of leaf damage. This hypothesis seems to have been confirmed by experiments with the rcd1 Arabidopsis mutant. This mutant showed higher ozone sensitivity associated with higher production of ethylene,18 and higher stomatal conductance than in wild-type plants during ozone exposure.85 However, questions still remain, because the ROS, which is generated with xanthine-xanthine oxidase superoxide-generating system independent of the stomata, triggered a higher level of ROS-dependent promotion of the development of leaf lesions only in the rcd1 mutant,18 indicating that the sensitivity of rcd1 to ozone could not result only from a higher influx of ozone caused by inhibition of ABA signaling by ethylene. Further experiments are thus necessary to understand whether ozone-induced ethylene production inhibits stomatal closure through its effects on ABA signaling, because previous studies showed mutually antagonistic interactions between ethylene and ABA signaling.84,86,87
Many studies, including some that have used Arabidopsis mutants that overproduce ethylene or transgenic plants that exhibit reduced ozone-induced ethylene production, have shown that ozone-induced ethylene promotes the development of leaf injury.5,20,88 On the other hand, a few reports show that ethylene suppresses leaf damage caused by ozone. For example, pretreatment with ethylene increases ozone tolerance in pea (Pisum sativum) and mung bean (Vigna radiata).89 A dual role for ethylene in ozone tolerance has also been observed in different genotypes of silver birch (Betula pendula Roth). An ozone-tolerant silver birch clone produced little ethylene in response to ozone treatment, and ethylene production was temporally occurred,90 whereas an ozone-sensitive clone generated a prolonged high level of ethylene. Therefore, ethylene can serve as a mediator of either survival or cell death, depending on its level of synthesis and the temporal pattern of its synthesis. In addition, ethylene signaling in the ozone-tolerant Arabidopsis ecotype Col-0 might result in ozone tolerance through the induction of many defense genes. Indeed, transcriptome analysis using Arabidopsis macroarray showed that the induction of many defense-related genes was suppressed in the ein2 Arabidopsis mutant.22 A recent study showed that low levels and temporal of ethylene evolution over time are necessary to promote the glutathione biosynthesis pathway that produces this ROS scavenger (Yoshida S, Tsukuba University, unpublished data). These results indicate that a low level of rapid but transient ethylene production is required for the acquisition of ozone tolerance in plants through the induction of many defense genes.
Jasmonic Acid Suppresses the Development of Lesions
Jasmonic acid is a derivative of the octadecanoid lipid pathway, in which it is derived from linolenic acid, and is a plant signaling compound involved in the regulation of many stress responses and of development.91 JA production is induced by a wide range of biotic and abiotic stresses, such as wounding, water deficits, and pathogen attack.91–96 JA is synthesized from linolenic acid through oxygenation by lipoxygenase (LOX), then converted into 12-oxo-phytodienoic acid by allene oxide synthase (AOS) and allene oxide cyclase (AOC) (Fig. 2). JA is also synthesized from 12-oxo-phytodienoic acid through reduction by 12-oxo-phytodienoic acid reductase (OPR) and three β-oxidation steps. Jasmonic acid carboxyl methyltransferase generates methyl jasmonate (MeJA). MeJA induces a set of genes for JA biosynthesis, including LOX2, AOS, AOC and OPR3, suggesting that MeJA is a key compound in the JA-signaling pathway, in which MeJA controls its own production by means of a positive feedback mechanism.97 Induction of JA biosynthesis by ozone was expected on the basis of the finding that the expression of WIN3.7 (wound-inducible trypsin inhibitor 3.7), which is induced by JA treatment, was increased by ozone exposure.98 This research group also showed that JA accumulated in ozone-exposed poplar.99 In addition, induction of LOX, AOS, and OPR (all involved in JA biosynthesis) was identified in ozone-treated plants.100–103 Based on these results, it is not surprising that JA or MeJA, as well as many other compounds derived from fatty acids, act as signaling molecules. The responses to these compounds have been designated as phyto-oxylipin cascades, which also induce defense responses against pathogens or other environmental stresses.104 Indeed, several recent studies have demonstrated that fatty acids in chloroplasts are involved in PCD in response to stressors, including ozone.105–107
The ozone sensitivity of JA-deficient mutants shows that JA might be involved in the repression of ROS-dependent lesion development in ozone-exposed leaves. The JA-insensitive Arabidopsis mutants methyl-jasmonate resistant1 (jar1),108 coronatine insensitive1 (coi1),109 and ozone-sensitive and jasmonate-insensitive 1 (oji1),21 and the JA-biosynthesis defective fad3/7/8 triple mutant and the 12-oxophytodienoate reductase 3 (opr3) mutant are all highly sensitive to ozone.18,102,110 The involvement of JA is further supported by studies that showed pretreatment with MeJA conferring ozone tolerance in tobacco and Arabidopsis.63,102 Inhibition of leaf damage by JA could be achieved partly through the regulation of ethylene receptors, because MeJA has been shown to induce a genes that encode an ethylene receptor (ERS2).111 The increased de novo synthesis of receptors has been proposed to decrease ethylene sensitivity.112 In this way, JA might inhibit the development of ethylene-dependent lesions caused by ozone. Another hypothesis for the protection against ozone-induced leaf damage by JA involves the coordinated activation of metabolic pathways for the production of antioxidants. Sasaki-Sekimoto et al.110 showed that the expression of genes involved in the metabolic pathways for the production of ascorbic acid (VTC1, VTC2, DHAR and MDHAR) and glutathione (GSH1 and GSH2) was induced by MeJA treatment. These authors also showed that JA induces the accumulation of ascorbic acid and glutathione, suggesting that the coordinated activation of metabolic pathways mediated by JA could provide resistance to ozone damage.
Crosstalk Among Ethylene, SA and JA in Ozone-Exposed Plants
Thus far, I have described the roles of SA, ethylene, and JA in ozone-exposed plants as though these substances act independently, but in reality, they act in mutually antagonistic or coordinated ways during ozone exposure. A JA-insensitive Arabidopsis mutant, jar1, accumulated a higher level of SA and showed a higher level of PR1 expression than wild-type plants.102 In addition, the ozone-sensitive Arabidopsis ecotype Cvi-0 is known to produce large amounts of SA in response to ozone treatment, and this plant shows low JA sensitivity. These results suggest that SA accumulation in response to ozone is negatively regulated by JA signaling. JA signaling also negatively regulates ethylene biosynthesis. The ozone-sensitive and jasmonate-semi-insensitive (oji1) Arabidopsis mutant showed ozone sensitivity, reduced JA sensitivity, and a high level of ethylene production.21 Treatment of this mutant with JA reduced ethylene production and decreased ozone sensitivity. Moreover, the higher level of ozone-induced ethylene production that occurs in the ozone-sensitive Arabidopsis mutant rcd1 is also decreased by treatment with JA.18 These results indicate that JA signaling also negatively regulates ethylene biosynthesis, and that JA could attenuate the ozone sensitivity that results from a high level of ethylene production.
The interaction between SA and ethylene has also been studied in ozone-exposed plants. An increase in ozone-inducible ethylene production was observed in plants pretreated with SA,20 and ethylene production in response to ozone decreased in a NahG transformed Arabidopsis that does not accumulate SA. These findings suggest that ethylene biosynthesis in response to ozone is regulated by SA signaling. However, another research group obtained contradictory results. Transgenic tobacco with inhibited ethylene biosynthesis generated lower levels of SA than the wild-type in response to ozone,24 suggesting that SA biosynthesis in response to ozone was regulated by ethylene signaling. This discrepancy might be explained by the level of production of ozone-induced ethylene in the plants. As described above, ozone-induced ethylene can play different roles, depending on its production level and the timing of production. A low and transient production of ethylene induces the expression of defense genes, resulting in the acquisition of ozone tolerance, whereas a high and prolonged production of ethylene promotes the development of leaf lesions in response to ozone. For example, the ozone-tolerant Arabidopsis ecotype Col-0 generates a low level of ethylene in response to ozone.5 In this case, ethylene decreases ozone-induced leaf damage through the induction of defense-related genes that are also induced by SA signaling.22 In other words, ethylene and SA signaling act cooperatively in this plant. Decreasing SA production or signaling in this plant decreases the induction of defense-related genes, which suggests that ethylene signaling is also decreased. Therefore, SA signaling seems to regulate ethylene signaling in this plant.
In contrast, the ozone-sensitive Arabidopsis mutant rcd1 accumulates high levels of both ethylene and SA.12,18 Coaccumulation of ethylene and SA was also observed in two Arabidopsis ethylene over-producer mutants, eto1 and eto3.20 Although these are examples of Arabidopsis mutants, other plants also produce high levels of ethylene, such as tobacco cultivar ‘Bel-W3’.4 In this tobacco, a marked increase in SA levels and increased activity of benzoic acid 2-hydroxylase, which catalyses SA biosynthesis, were also observed.113 It is well known that ethylene and SA together promote continuous ROS production and cell death in response to ozone.19 In these cases, decreasing SA production might be accompanied by decreasing ethylene biosynthesis. Thus, it seems that ethylene signaling regulates SA production in these plants. To understand the interaction between ethylene and SA in ozone-exposed plants, it is important to clarify whether or not the plants used in each experiment produce a high level of ethylene. Currently, it seems that ozone-induced ethylene depends on SA and vice versa.
As described previously, the development of ozone-induced lesions in leaves seems to be under hormonal control, with different phytohormones and their interactions regulating ROS production and the competence of the cell to perceive and react to ROS signals.19 Regulation of ROS-dependent cell death in the oxidative cell death cycle has been proposed on the basis of work with plants undergoing HR,114 and this hypothesis was subsequently modified on the basis of ozone-induced oxidative cell death.18,19 In this model, ROS, SA, ethylene and cell death function in a self-amplifying feed-forward loop in the regulation of cell death. JA is involved in the containment of lesion development. According to this model, the levels of SA, ethylene, and JA production depend on the magnitude of ROS generation, and ozone-induced cell death is then regulated by the functions of these phytohormones. Cell death leads to additional ROS production, resulting in further production of phytohormones. This model is very simple, but it can explain a number of the observations of responses induced by ozone. However, both SA and ethylene can also protect plants from oxidative stresses, as described above. Thus, this model can explain the amplification of ozone-induced lesion development by phytohormones, but cannot account for the protective function of optimal levels of SA and ethylene, which are known to induce defense responses.
Interactions Among Phytohormones in Ozone-Exposed Plants
The knowledge covered in this review article support the notion that ozone-induced cell death is the result of cross-talk between various signaling pathways. Based on these observations, I have developed a hypothetical model that illustrates the complexity of the multiple interacting signaling pathways that determine the magnitude of the ozone-induced defense responses and cell death (Fig. 4). This model is based on the model of hormonal interactions that regulate ROS-dependent cell death that was proposed by Overmyer et al.18,19 On entering the leaf tissue through stomata, ozone reacts primarily with phenolic and olefinic compounds in plant cell walls and with unsaturated lipids in plasma membranes, generating ROS. These ozone-derived ROS mimic an oxidative burst in plant cells, and the perception of this change triggers a wide array of signaling cascades similar to those induced by plant pathogens. Furthermore, ozone-induced changes in levels of free calcium may phosphorylate one of the subunits of NADPH-oxidase known to generate ROS, or it may directly affect to the NADPH oxidase activity because this enzyme has an N-terminal sequence with two calcium-binding EF-hand motifs.47,115 The generated ROS activate MAPKs (such as SIPK, MPK6 and MPK3) within 10 to 30 minutes. Among these MAPKs, MPK6 contributes to ethylene production through its effect on the accumulation of ACS6 and, thus, on elevated levels of cellular ACS activity. The production of SA and JA is also induced by ROS. Further, PCD occurs where contact with ozone has occurred. After the production of phytohormones, the fate of ozone-exposed cells then depends on the levels of ethylene and SA accumulation.
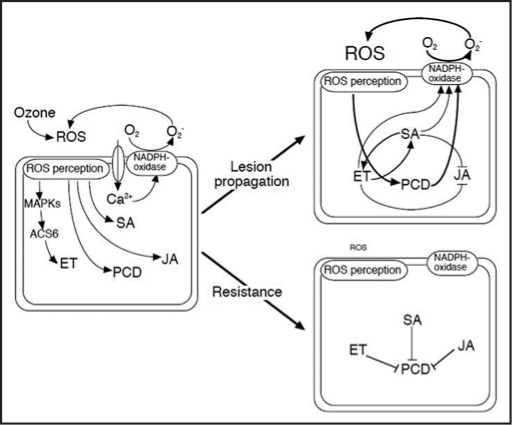
Figure 4.
A hypothetical model for the interactions among the salicylic acid, ethylene, and jasmonic acid signaling pathways that modulate the ozone-induced propagation of leaf lesions or resistance to these lesions. ROS, reactive oxygen species; MAPK, mitogen-activated protein kinase; ACS, 1-aminocyclopropane-1-carboxylic acid synthase; ET, ethylene; SA, salicylic acid; JA, jasmonic acid; PCD, programmed cell death.
Cells in which lesions have developed produce high levels of SA and ethylene, generating additional ROS through activation of NADPH-oxidase. SA and ethylene biosynthesis and signaling enhance each other, but inhibit the JA signaling that could provide ozone tolerance via the activation of the cell's defense responses. Consequently, ROS generation increases exponentially as a result of the oxidative cell death cycle, and visible leaf lesions appear as the damage spreads. On the other hand, if optimal SA and ethylene levels exist, ozone-exposed cells resist ROS. Optimal levels of SA and ethylene production induce the expression of defense-related genes that are involved in antioxidant and detoxification processes. The JA signaling pathway, which induces a series of genes responsible for biosynthesis of ascorbic acid and glutathione, is also active in these cells. Therefore, SA, ethylene, and JA signals interact to determine the promotion or inhibition of PCD in response to ozone exposure.
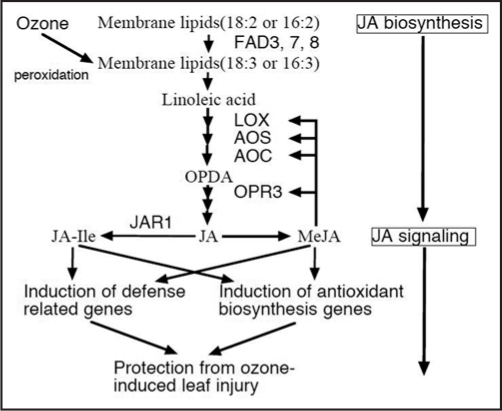
Figure 3.
Proposed pathways for jasmonic acid biosynthesis and signaling in ozone-exposed plants. Jasmonic acid (JA) biosynthesis in ozone-exposed plants is initiated from peroxodation of membrane lipids. Synthesized JA is further metabolized into methyl JA (MeJA) or JA-isoleucine (JA-Ile). These JA derived conjugates contribute protection from ozone-induced leaf damage through induction of defense related and/or antioxidant biosynthesis genes.
Abbreviations
- ACC
1-aminocyclopropane-1-carboxylic acid
- ACO
ACC oxidase
- ACS
ACC synthase
- AOC
allene oxide cyclase
- AOS
allene oxide synthase
- GST
glutathione-S-transferase
- ICS
isochorismate synthase
- JA
jasmonic acid
- LOX
lipoxygenase
- MAPK
mitogen-activated protein kinase
- MeJA
methyl jasmonate
- OPR
12-oxo-phytodienoic acid reductase
- PAL
phenylalanine ammonia-lyase
- PCD
programmed cell death
- PR
pathogenesis-related
- ROS
reactive oxygen species
- SA
salicylic acid
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/5538
References
- 1.Krupa SV, Manning WJ. Atmospheric ozone: Formation and effect on vegetation. Environ Pollut. 1988;50:101–137. doi: 10.1016/0269-7491(88)90187-x. [DOI] [PubMed] [Google Scholar]
- 2.Pell EJ, Schlangnhaufer CD, Arteca RN. Ozone-induced oxidative stress: Mechanism of action and reaction. Physiol Plant. 1997;100:264–273. [Google Scholar]
- 3.Kadono T, Yamaguchi Y, Furuichi T, Hirono M, Garrec JP, Kawano T. Ozone-induced cell death mediated with oxidative and calcium signaling pathways in tobacco Bel-W3 and Bel-B cell suspension cultures. Plant Signal Behav. 2006;1:312–322. doi: 10.4161/psb.1.6.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heggestad HE. Origin of BelW3, BelC and BelB tobacco varieties and their use as indicators of ozone. Environ Pollut. 1991;74:264–291. doi: 10.1016/0269-7491(91)90076-9. [DOI] [PubMed] [Google Scholar]
- 5.Tamaoki M, Matsuyama T, Kanna M, Nakajima N, Kubo A, Aono M, Saji H. Differential ozone sensitivity among Arabidopsis accessions and its relevance to ethylene synthesis. Planta. 2003;216:552–560. doi: 10.1007/s00425-002-0894-2. [DOI] [PubMed] [Google Scholar]
- 6.Laloi C, Apel K, Danon A. Reactive oxygen signalling: The latest news. Curr Opin Plant Biol. 2004;7:323–328. doi: 10.1016/j.pbi.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Noctor G, Foyer CH. Ascorbate and glutathione: Keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 8.Foyer CH, Lopez-Delgado H, Dat JF, Scott IM. Hydrogen peroxide and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol Plant. 1997;100:241–254. [Google Scholar]
- 9.Polle A. Dissection the superoxide dismutase-ascorbate-glutathione pathway by metabolic modeling: Computer analysis as a step towards flux analysis. Plant Physiol. 2001;126:445–462. doi: 10.1104/pp.126.1.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kangasjärvi J, Talvinen J, Utriainen M, Karjalainen R. Plant defence systems induced by ozone. Plant Cell Environ. 1994;17:783–794. [Google Scholar]
- 11.Kangasjärvi J, Jaspars P, Kollist H. Signaling and cell death in ozone-exposed plants. Plant Cell Environ. 2005;28:1–16. [Google Scholar]
- 12.Overmyer K, Brosch M, Pellinen R, Kuittinen T, Tuominen H, Ahlfors R, Keinanen M, Saarma M, Scheel D, Kangasjärvi J. Ozone-induced programmed cell death in the Arabidopsis radical-induced cell death1 mutant. Plant Physiol. 2005;137:1092–1104. doi: 10.1104/pp.104.055681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao MV, Davis KR. The physiology of ozone induced cell death. Planta. 2001;213:682–690. doi: 10.1007/s004250100618. [DOI] [PubMed] [Google Scholar]
- 14.Sharma YK, Davis KR. The effect of ozone on antioxidant responses in plants. Free Radical Biol Med. 1997;23:480–488. doi: 10.1016/s0891-5849(97)00108-1. [DOI] [PubMed] [Google Scholar]
- 15.Sandermann H, Ernst D, Heller W, Langebertles C. Ozone: An abiotic elicitor of plant defense reaction. Trends Plant Sci. 1998;3:47–50. [Google Scholar]
- 16.Dong X, SA JA. Ethylene, and disease resistance in plants. Curr Opin Plant Biol. 1998;1:316–323. doi: 10.1016/1369-5266(88)80053-0. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg JT, Silverman FP, Liang H. Uncoupling salicylic acid-dependent cell death and defence-related responses from disease resistance in the Arabidopsis mutant. Genetics. 2000;156:341–350. doi: 10.1093/genetics/156.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Overmyer K, Tuominen H, Kettunen R, Betz C, Langebartels C, Sandermann H, Jr, Kangasjärvi J. The ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. Plant Cell. 2000;12:1849–1862. doi: 10.1105/tpc.12.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Overmyer K, Brosch M, Kangasjärvi J. Reactive oxygen species and hormonal control of cell death. Trends Plant Sci. 2003;8:335–342. doi: 10.1016/S1360-1385(03)00135-3. [DOI] [PubMed] [Google Scholar]
- 20.Rao MV, Lee H, Davis KR. Ozone-induced ethylene production is dependent on salicylic acid, and both salicylic acid and ethylene act in concert to regulate ozone-induced cell death. Plant J. 2002;32:447–456. doi: 10.1046/j.1365-313x.2002.01434.x. [DOI] [PubMed] [Google Scholar]
- 21.Kanna M, Tamaoki M, Kubo A, Nakajima N, Rakwal R, Agrawal GK, Tamogami S, Ioki M, Ogawa D, Saji H, Aono M. Isolation of an ozone-sensitive and jasmonate-semi-insensitive Arabidopsis mutant (oji1) Plant Cell Physiol. 2003;44:1301–1310. doi: 10.1093/pcp/pcg157. [DOI] [PubMed] [Google Scholar]
- 22.Tamaoki M, Nakajima N, Kubo A, Aono M, Matsuyama T, Saji H. Transcriptome analysis of O3-exposed Arabidopsis reveals that multiple signal pathways act mutually antagonistically to induce gene expression. Plant Mol Biol. 2003;53:443–456. doi: 10.1023/B:PLAN.0000019064.55734.52. [DOI] [PubMed] [Google Scholar]
- 23.Tuomainen J, Overmyer K, Keinänen M, Kollist H, Kangasjärvi J. Mutual antagonism of ethylene and jasmonic acid regulates ozone-induced spreading cell death in Arabidopsis. Plant J. 2004;39:59–69. doi: 10.1111/j.1365-313X.2004.02107.x. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa D, Nakajima N, Sano T, Tamaoki M, Aono M, Kubo A, Kanna M, Ioki M, Kamada H, Saji H. Salicylic acid accumulation under O3 exposure is regulated by ethylene in tobacco plants. Plant Cell Physiol. 2005;46:1062–1072. doi: 10.1093/pcp/pci118. [DOI] [PubMed] [Google Scholar]
- 25.Laisk A, Kull O, Moldau H. Ozone concentration in leaf intracellular spaces is close to zero. Plant Physiol. 1989;90:1163–1167. doi: 10.1104/pp.90.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mudd T. Biochemical basis for the toxicity of ozone. In: Yunus M, Iqbal M, editors. Plant Response to Air Pollution. New York, NY: John Wiley; 1997. pp. 267–284. [Google Scholar]
- 27.Shinozaki K, Yamaguchi-Shinozaki K. Gene expression and signal transduction in water-stress response. Plant Physiol. 1997;115:327–334. doi: 10.1104/pp.115.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang C, Shockey JA. The ethylene-response pathway: Signal perception to gene regulation. Curr Opin Plant Biol. 1999;2:352–358. doi: 10.1016/s1369-5266(99)00004-7. [DOI] [PubMed] [Google Scholar]
- 29.McDowell JM, Dangl JL. Signal transduction in the plant immune response. Trends Biochem Sci. 2000;25:79–82. doi: 10.1016/s0968-0004(99)01532-7. [DOI] [PubMed] [Google Scholar]
- 30.Knight H, Knight MR. Abiotic stress signalling pathways: Specificity and cross-talk. Trends Plant Sci. 2001;6:262–267. doi: 10.1016/s1360-1385(01)01946-x. [DOI] [PubMed] [Google Scholar]
- 31.Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamattina L, Garcia-Mata C, Graziano M, Pagnussat G. Nitric oxide: The versatility of an extensive signal molecule. Annu Rev Plant Biol. 2003;54:109–136. doi: 10.1146/annurev.arplant.54.031902.134752. [DOI] [PubMed] [Google Scholar]
- 33.Davis R. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 34.Chang L, Karin M. Mammalian MAP kinase signaling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 35.Innes RW. Mapping out the roles of MAP kinases in plant defense. Trends Plant Sci. 2001;6:392–394. doi: 10.1016/s1360-1385(01)02058-1. [DOI] [PubMed] [Google Scholar]
- 36.Zhang S, Klessig DF. MAPK cascades in plant defense signaling. Trends Plant Sci. 2001;6:520–527. doi: 10.1016/s1360-1385(01)02103-3. [DOI] [PubMed] [Google Scholar]
- 37.Jonak C, Ökrész L, Bögre L, Hirt H. Complexity, crosstalk and integration of plant MAP kinase signalling. Curr Opin Plant Biol. 2002;5:415–424. doi: 10.1016/s1369-5266(02)00285-6. [DOI] [PubMed] [Google Scholar]
- 38.Samuel MA, Miles GP, Ellis BE. Ozone treatment rapidly activates MAP kinase signaling in plants. Plant J. 2000;22:367–376. doi: 10.1046/j.1365-313x.2000.00741.x. [DOI] [PubMed] [Google Scholar]
- 39.Ahlfors R, Macioszek V, Rudd J, Brosché M, Schlichting R, Scheel D, Kangasjärvi J. Stress hormone-independent activation and nuclear translocation of mitogen-activated protein kinases in Arabidopsis thaliana during ozone exposure. Plant J. 2004;40:512–522. doi: 10.1111/j.1365-313X.2004.02229.x. [DOI] [PubMed] [Google Scholar]
- 40.Ren D, Yang H, Zhang S. Cell death mediated by MAPK is associated with hydrogen peroxide production in Arabidopsis. J Biol Chem. 2002;277:559–565. doi: 10.1074/jbc.M109495200. [DOI] [PubMed] [Google Scholar]
- 41.Samuel MA, Brian E, Ellis BE. Double jeopardy: Both overexpression and suppression of a redox-activated plant mitogen-activated protein kinase render tobacco plants ozone sensitive. Plant Cell. 2002;14:2059–2069. doi: 10.1105/tpc.002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomi K, Ogawa D, Katou S, Kamada H, Nakajima N, Saji H, Soyano T, Sasabe M, Machida Y, Matsuhara H, Ohashi Y, Seo S. A mitogen-activated protein kinase NtMPK4 activated by SIPKK is required for jasmonic acid signaling and involved in ozone tolerance via stomatal movement in tobacco. Plant Cell Physiol. 2005;46:1902–1914. doi: 10.1093/pcp/pci211. [DOI] [PubMed] [Google Scholar]
- 43.Sanders D, Pelloux J, Brownlee C, Harper JF. Calcium at the crossroads of signaling. Plant Cell. 2002;14:S401–S417. doi: 10.1105/tpc.002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clayton H, Knight MR, Knight H, McAinsh MR, Hetherington AM. Dissection of the ozone-induced calcium signature. Plant J. 1999;17:575–579. doi: 10.1046/j.1365-313x.1999.00411.x. [DOI] [PubMed] [Google Scholar]
- 45.Evans NH, McAinsh MR, Hetherington AM, Knight MR. ROS perception in Arabidopsis thaliana: The ozone-induced calcium response. Plant J. 2005;41:615–626. doi: 10.1111/j.1365-313X.2004.02325.x. [DOI] [PubMed] [Google Scholar]
- 46.Miles GP, Samuel MA, Jones AM, Ellis BE. Mastoparan rapidly activates plant MAP kinase signaling independent of heterotrimeric G proteins. Plant Physiol. 2004;134:1332–1336. doi: 10.1104/pp.103.037275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keller T, Damude HG, Werner D, Doerner P, Dixon RA, Lamb C. A plant homologue of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell. 1998;10:255–266. doi: 10.1105/tpc.10.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yalpani N, Enyedi AJ, Leo J, Raskin I. Ultraviolet-light and ozone stimulate accumulation of salicylic-acid, pathogenesis-related protein and virus-resistance in tobacco. Planta. 1994;193:372–376. [Google Scholar]
- 49.Leon J, Yalpani N, Raskin I, Lawton MA. Induction of benzoic acid 2-hydroxylase in virus-inoculated tobacco. Plant Physiol. 1993;103:323–328. doi: 10.1104/pp.103.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yalpani N, Altier DJ, Barbour E, Cigan AL, Scelonge CJ. Production of 6-methylsalicylic acid by expression of a fungal polyketide synthase activates disease resistance in tobacco. Plant Cell. 1993;13:1401–1409. doi: 10.1105/tpc.13.6.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mauch-Mani B, Slusarenko AJ. Production of salicylic acid precursors is a major function of phenylalanine ammonia-lyase in the resistance of Arabidopsis to Peronospora parasitica. Plant Cell. 1996;8:203–212. doi: 10.1105/tpc.8.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coquoz J, Buchala A, Metraux JP. The biosynthesis of salicylic acid in potato plants. Plant Physiol. 1998;117:1095–1101. doi: 10.1104/pp.117.3.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ribnicky DM, Shulaev V, Raskin I. Intermediates of salicylic acid biosynthesis in tobacco. Plant Physiol. 1998;118:565–572. doi: 10.1104/pp.118.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bate NJ, Orr J, Ni W, Meromi A, Nadler-Hassar T, Doerner PW, Dixon RA, Lamb CJ, Elkind Y. Quantitative relationship between phenylalanine ammonia-lyase levels and phenylpropanoid accumulation in transgenic tobacco identifies a rate-determining step in natural product synthesis. Proc Natl Acad Sci USA. 1994;91:7608–7612. doi: 10.1073/pnas.91.16.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Howles PA, Sewalt V, Paiva NL, Elkind Y, Bate NJ, Lamb C, Dixon RA. Overexpression of L-phenylalanine ammonia-lyase in transgenic tobacco plants reveals control points for flux into phenylpropanoid biosynthesis. Plant Physiol. 1996;112:1617–1624. doi: 10.1104/pp.112.4.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pallas JA, Paiva LN, Lamb C, Dixon AR. Tobacco plants epigenetically suppressed in phenylalanine ammonia-lyase expression do not develop systemic acquired resistance in response to infection by tobacco mosaic virus. Plant J. 1996;10:281–293. [Google Scholar]
- 57.Wildermuth MC, Dewdney J, Wu G, Ausubel FM. Isochorismate synthase is required to synthesize salicylic acid for plant defense. Nature. 2001;414:562–565. doi: 10.1038/35107108. [DOI] [PubMed] [Google Scholar]
- 58.Gaille C, Reimmann C, Haas D. Isochorismate synthase (PchA), the first and rate-limiting enzyme in salicylate biosynthesis of Pseudomonas aeruginosa. J Biol Chem. 2003;278:16893–16898. doi: 10.1074/jbc.M212324200. [DOI] [PubMed] [Google Scholar]
- 59.Nawrath C, Metraux JP. Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell. 1999;11:1393–1404. doi: 10.1105/tpc.11.8.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Tegelen LJ, Moreno PR, Crose AF, Verpoorte R, Wullems GJ. Purification and cDNA cloning of isochorismate synthase from elicited cell cultures of Catharanthus roseus. Plant Physiol. 1999;119:705–712. doi: 10.1104/pp.119.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ogawa D, Nakajima N, Tamaoki M, Aono M, Kubo A, Kamada H, Saji H. The isochorismate pathway is negatively regulated by salicylic acid signaling in O3-exposed Arabidopsis. Planta. 2007;226:1277–1285. doi: 10.1007/s00425-007-0556-5. [DOI] [PubMed] [Google Scholar]
- 62.Geffney T, Friedrich L, Vernooij B, Nye G, Uknes S, Ward E, Kessmann H, Ryals J. Requirement of salicylic acid for the induction of systemic acquired resistance. Science. 1993;261:754–756. doi: 10.1126/science.261.5122.754. [DOI] [PubMed] [Google Scholar]
- 63.Örvar BL, McPherson J, Ellis BE. Preactivating wounding response in tobacco prior to high-level ozone exposure prevents necrotic injury. Plant J. 1997;11:203–212. doi: 10.1046/j.1365-313x.1997.11020203.x. [DOI] [PubMed] [Google Scholar]
- 64.Rao MV, Davis KR. Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: The role of salicylic acid. Plant J. 1999;17:603–614. doi: 10.1046/j.1365-313x.1999.00400.x. [DOI] [PubMed] [Google Scholar]
- 65.Mazel A, Levine A. Induction of cell death in Arabidopsis by superoxide in combination with salicylic acid or with protein synthesis inhibitors. Free Radic Bio Med. 2001;30:98–106. doi: 10.1016/s0891-5849(00)00452-4. [DOI] [PubMed] [Google Scholar]
- 66.Sharma YK, León J, Raskin I, Davis KR. Ozone-induced responses in Arabidopsis thaliana: The role of salicylic acid in the accumulation of defense-related transcripts and induced resistance. Proc Natl Acad Sci USA. 1996;93:5099–5104. doi: 10.1073/pnas.93.10.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zarembinski TI, Theologis A. Ethylene biosynthesis and action: A case of conservation. Plant Mol Biol. 1994;26:1579–1597. doi: 10.1007/BF00016491. [DOI] [PubMed] [Google Scholar]
- 68.Johnson PR, Ecker JR. The ethylene gas signal transduction pathway: A molecular perspective. Annu Rev Genet. 1998;32:227–254. doi: 10.1146/annurev.genet.32.1.227. [DOI] [PubMed] [Google Scholar]
- 69.Bleecker AB, Kende H. Ethylene: A gaseous signal molecule in plants. Annu Rev Cel Dev Biol. 2000;16:1–18. doi: 10.1146/annurev.cellbio.16.1.1. [DOI] [PubMed] [Google Scholar]
- 70.Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol. 1984;35:155–189. [Google Scholar]
- 71.Kende H. Ethylene biosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:283–307. [Google Scholar]
- 72.Tuomainen J, Betz C, Kangasjärvi J, Ernst D, Yin ZH, Langebartels C, Sandermann H., Jr Ozone induction of ethylene emission in tomato plants: Regulation by differential accumulation of transcripts for the biosynthetic enzymes. Plant J. 1997;12:1151–1162. [Google Scholar]
- 73.Schlagnhaufer CD, Arteca RN, Pell EJ. Sequential expression of two 1-aminocyclopropane-1-carboxylate synthase genes in response to biotic and abiotic stress in potato leaves. Plant Mol Biol. 1997;35:683–688. doi: 10.1023/a:1005857717196. [DOI] [PubMed] [Google Scholar]
- 74.Tsuchisaka A, Theologis A. Unique and overlapping expression patterns among the Arabidopsis 1-amino-cyclopropane-1-carboxylate synthase gene family members. Plant Physiol. 2004;136:2982–3000. doi: 10.1104/pp.104.049999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vahala J, Schlagnhaufer CD, Pell EV. Induction of an ACC synthase cDNA by ozone in light grown Arabidopsis thaliana leaves. Physiol Plant. 1998;103:45–50. [Google Scholar]
- 76.Liu Y, Zhang S. Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell. 2004;16:3386–3399. doi: 10.1105/tpc.104.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Craker L. Ethylene production from ozone injured plants. Environ Pollut. 1971;1:299–304. [Google Scholar]
- 78.Tingey DT, Standly C, Field RW. Stress ethylene evolution: A measure of ozone effects on plants. Atmos Environ. 1976;10:969–974. doi: 10.1016/0004-6981(76)90204-3. [DOI] [PubMed] [Google Scholar]
- 79.Langebartels C, Kerner K, Leonardi S, Schraudner M, Trost M, Heller W, Sandermann H., Jr Biochemical plant responses to ozone. Plant Physiol. 1991;95:882–889. doi: 10.1104/pp.95.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mehlhorn H, Oshea JM, Wellburn AR. Atmospheric ozone interacts with stress ethylene formation by plants to cause visible plant injury. J Exp Bot. 1991;42:17–24. [Google Scholar]
- 81.Elstner EF, Osswald W, Youngman RJ. Basic mechanisms of pigment bleaching and loss of structural resistance in spruce (Picea abies) needles: Advances in phytomedical diagnostics. Experientia. 1985;41:591–597. [Google Scholar]
- 82.Mehlhorn H, Wellburn AR. Stress ethylene formation determines plant sensitivity to ozone. Nature. 1987;327:417–418. [Google Scholar]
- 83.Bae GY, Nakajima N, Ishizuka K, Kondo N. The role in ozone phytotoxicity of the evolution of ethylene upon induction of 1-aminocyclopropane-1-carboxylic acid synthase by ozone fumigation in tomato plants. Plant Cell Physiol. 1996;37:129–134. [Google Scholar]
- 84.Tanaka Y, Sano T, Tamaoki M, Nakajima N, Kondo N, Hasezawa S. Ethylene inhibits the abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol. 2005;138:2337–2343. doi: 10.1104/pp.105.063503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ahlfors R, Lång S, Overmyer K, Jaspers P, Brosché M, Tauriainen A, Kollist H, Tuominen H, Belles-Boi E, Piippo M, Inzé DE, Palva ET, Kangasjärvi J. Arabidopsis RADICAL-INDUCED CELL DEATH1 belongs to the WWE protein-protein interaction domain protein family and modulates abscisic acid, ethylene, and methyl jasmonate responses. Plant Cell. 2004;16:1925–1937. doi: 10.1105/tpc.021832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beaudoin N, Serizet C, Gosti F, Giraudat J. Interactions between abscisic acid and ethylene signaling cascades. Plant Cell. 2000;12:1103–1115. doi: 10.1105/tpc.12.7.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P. Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell. 2000;12:1117–1126. doi: 10.1105/tpc.12.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nakajama N, Itoh T, Takikawa S, Asai N, Tamaoki M, Aono M, Kubo A, Azumi Y, Kamada H, Saji H. Improvement in ozone tolerance of tobacco plants with an antisense DNA for 1-aminocyclopropane-1-carboxylase synthase. Plant Cell Environ. 2002;25:727–735. [Google Scholar]
- 89.Mehlhorn H. Ethylene-promoted ascorbate peroxidase activity protects plants against hydrogen peroxide, ozone and paraquat. Plant Cell Environ. 1990;13:971–976. [Google Scholar]
- 90.Vahala J, Ruonala R, Keinänen M, Tuominen H, Kangasjärvi J. Ethylene insensitivity modulates ozone-induced cell death in birch. Plant Physiol. 2003;132:185–195. doi: 10.1104/pp.102.018887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Turner JG, Ellis C, Devoto A. The jasmonate signal pathway. Plant Cell. 2002;14:S153–S164. doi: 10.1105/tpc.000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Creelman RA, Mullet JE. Oligosaccharins, brassinolides, and jasmonates: Nontraditional regulators of plant growth, development, and gene expression. Plant Cell. 1997;9:1211–1223. doi: 10.1105/tpc.9.7.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pieterse CM, van Wees SC, van Pelt JA, Knoester M, Laan R, Gerrits H, Weisbeek PJ, van Loon LC. A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell. 1998;10:1571–1580. doi: 10.1105/tpc.10.9.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Staswick PE, Yuen GY, Lehman CC. Jasmonate signaling mutants of Arabidopsis are susceptible to the soil fungus Pythium irregulare. Plant J. 1998;15:747–754. doi: 10.1046/j.1365-313x.1998.00265.x. [DOI] [PubMed] [Google Scholar]
- 95.Farmer EE, Almeras E, Krishnamurthy V. Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Curr Opin Plant Biol. 2003;6:372–378. doi: 10.1016/s1369-5266(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 96.Rojo E, Solano R, Sanchez-Serrano JJ. Interactions between signaling compounds involved in plant defense. J Plant Growth Regul. 2003;22:82–98. [Google Scholar]
- 97.Sasaki Y, Asamizu E, Shibata D, Nakamura Y, Kaneko T, Awai K, Amagai M, Kuwata C, Tsugane T, Masuda T, Shimada H, Takamiya K, Ohta H, Tabata S. Monitoring of methyl jasmonate-responsive genes in Arabidopsis by cDNA macroarray: Self-activation of jasmonic acid biosynthesis and crosstalk with other phytohormone signaling pathways. DNA Res. 2001;8:153–161. doi: 10.1093/dnares/8.4.153. [DOI] [PubMed] [Google Scholar]
- 98.Koch JR, Scherzer AJ, Eshita SM, Davis KR. Ozone sensitivity in hybrid poplar is correlated with a lack of defense-gene activation. Plant Physiol. 1998;118:1243–1252. doi: 10.1104/pp.118.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koch JR, Creelman RA, Eshita SM, Seskar M, Mullet JE, Davis KR. Ozone sensitivity in hybrid poplar correlates with insensitivity to both salicylic acid and jasmonic acid: The role of programmed cell death in lesion formation. Plant Physiol. 2000;123:487–496. doi: 10.1104/pp.123.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maccarrone M, Veldink GA, Vliegenthart JFC. Thermal injury and ozone stress affect soybean lipoxygenases expression. FEBS Lett. 1992;309:225–230. doi: 10.1016/0014-5793(92)80778-f. [DOI] [PubMed] [Google Scholar]
- 101.Maccarrone M, Veldink GA, Vliegenthart JFC, Agrò AF. Ozone stress modulates amine oxidase and lipoxygenase expression in lentil (Lens culinaris) seedlings. FEBS Lett. 1997;408:241–244. doi: 10.1016/s0014-5793(97)00433-x. [DOI] [PubMed] [Google Scholar]
- 102.Rao MV, Lee H, Creelman RA, Mullet JE, Davis KR. Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. Plant Cell. 2000;12:1633–1646. doi: 10.1105/tpc.12.9.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mahalingam R, Shah N, Scrymgeour A, Fedoroff N. Temporal evolution of the Arabidopsis oxidative stress response. Plant Mol Biol. 2005;57:709–730. doi: 10.1007/s11103-005-2860-4. [DOI] [PubMed] [Google Scholar]
- 104.Blée E. Impact of phyto-oxylipins in plant defense. Trends Plant Sci. 2002;7:315–322. doi: 10.1016/s1360-1385(02)02290-2. [DOI] [PubMed] [Google Scholar]
- 105.Kachroo P, Shanklin J, Shah J, Whittle EJ, Klessig DF. A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proc Natl Acad Sci USA. 2001;98:9448–9453. doi: 10.1073/pnas.151258398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nandi A, Welti R, Shah J. The Arabidopsis thaliana dihydroxyacetone phosphate reductase gene SUPPRESSOR OF FATTY ACID DESATURASE DEFICIENCY1 is required for glycerolipid metabolism and for the activation of systemic acquired resistance. Plant Cell. 2004;16:465–477. doi: 10.1105/tpc.016907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yaeno T, Matsuda O, Iba K. Role of chloroplast trienoic fatty acids in plant disease defense responses. Plant J. 2004;40:931–941. doi: 10.1111/j.1365-313X.2004.02260.x. [DOI] [PubMed] [Google Scholar]
- 108.Staswick PE, Su W, Howell SH. Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc Natl Acad Sci USA. 1992;89:6837–6840. doi: 10.1073/pnas.89.15.6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Feys B, Benedetti CE, Penfold CN, Turner JG. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell. 1994;6:751–759. doi: 10.1105/tpc.6.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sasaki-Sekimoto Y, Taki N, Obayashi T, Aono M, Matsumoto F, Sakurai N, Suzuki H, Yokota-Hirai M, Noji M, Saito K, Masuda T, Takamiya K, Shibata D, Ohta H. Coordinated activation of metabolic pathways for antioxidants and defence compounds by jasmonates and their roles in stress tolerance in Arabidopsis. Plant J. 2005;44:653–668. doi: 10.1111/j.1365-313X.2005.02560.x. [DOI] [PubMed] [Google Scholar]
- 111.Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, Somerville SC, Manners JM. Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci USA. 2000;97:11655–11660. doi: 10.1073/pnas.97.21.11655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ciardi J, Klee H. Regulation of ethylene-mediated responses at the level of receptor. Ann Bot. 2001;88:813–822. [Google Scholar]
- 113.Pasqualini S, Torre GD, Ferranti F, Ederli L, Piccioni C, Reale L, Antonielli M. Salicylic acid modulates ozone-induced hypersensitive cell death in tobacco plants. Physiol Plant. 2002;115:204–212. doi: 10.1034/j.1399-3054.2002.1150205.x. [DOI] [PubMed] [Google Scholar]
- 114.Van Camp W, Van Montagu M, Inzè D. H2O2 and NO: Redox signals in disease resistance. Trends Plant Sci. 1998;3:330–334. [Google Scholar]
- 115.Torres MA, Onouchi H, Hamada S, Machida C, Hammond-Kosack KE, Jones JD. Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox) Plant J. 1998;14:365–370. doi: 10.1046/j.1365-313x.1998.00136.x. [DOI] [PubMed] [Google Scholar]