Abstract
Hydrogen peroxide (H2O2) promotes seed germination of cereal plants and ascorbic acid which acts as antioxidant suppresses the germination of wheat seeds, but the role of H2O2 scavenging on germination during seed maturation has not been demonstrated. We investigated relationship of germination, ascorbate, H2O2 scavenging enzymes and sensitivity to ascorbic acid (AsA) maturing seeds of two typical wheat (Triticum aestivum L.) cultivars, cvs. Shirogane-Komugi and Norin61. Shirogane-Komugi had marked high germination ability than Norin61 during seed maturation. Although the H2O2 content had no difference in the two culti-vars, sensitivity to AsA of Norin61 seeds was higher than that of Shirogane-Komugi seeds during seed maturation. The sensitivity to AsA closely correlated with germination characteristic in the two cultivars. Especially, at 28 days after pollination (DAP), sensitivity to AsA in Norin61 seeds was remarkably high. At that stage, no significant differences were observed in endogenous AsA level, ascorbate peroxidase (APX, EC 1.11.1.11) and dehydroascorbate reductase (DHAR, EC 1.8.5.1) activities in the two cultivars. However, catalase (CAT, EC 1.11.1.6) activity and CAT mRNA in Norin61 were remarkably higher than in Shirogane-Komugi. Sensitivity to AsA at 35 and 42 DAPs kept high levels in Norin61, and endogenous AsA and CAT activity in the seeds were significantly higher than in Shirogane-Komugi. These results revealed a direct correlation between germination and antioxidant sensitivity during the developmental stages of wheat seeds.
Key words: ascorbic acid, germination, hydorogen peroxide, maturation, wheat seed
Introduction
Germination and dormancy are an important mechanism in seed physiology and is a complex trait, influenced by a myriad of genetic and environmental factors that interact to maximize the long-term chance of survival of the seed.1 Abscisic acid (ABA) has been demonstrated to play an important role in regulating seed maturation and germination. During seed maturation, ABA content increases and regulates many key processes including the imposition and maintenance of germination and dormancy.2 In wheat, the strongest dormancy is associated with a red seed coat color, whereas the lines with white seed coats are nondormant or weakly dormant and therefore are susceptible to preharvest sprouting damage.3,4 Although Himi et al.5 reported that the seed color gene might enhance grain dormancy by increasing the sensitivity of embryos to ABA, the dormancy effect conferred by seed coat color was not large, because there was no difference in the amount of inhibitors between red and white-seed bran. Weidner and Paprocka6 proposed that dormancy of cereal caryopses might be at least partially controlled by the high level of free phenolic acids in seed, through their inhibitory effect on germination and cell division.
It is well known that many phenolic compounds in plant tissues are potential antioxidants: flavonoids, tannins and lignin precursors may work as reactive oxygen species (ROS) scavenging compounds. ROS such as hydroxyl radical (·OH), O2- and H2O2 in seed physiology are usually considered as toxic molecules.7 However, exogenously applied H2O2 ameliorates seed germination in many plants.8,9 This has been explained by the fact that the scavenging activity for H2O2 is sufficiently high, resulting in the production of O2 for mitochondrial respiration. In contrast, H2O2 promoted seed germination in a dose-dependent manner as did respiratory inhibitors, indicating that H2O2 itself possibly promotes seed germination rather than O2.10
Antioxidants which act as ROS scavenger in seed biology play a very important role in the growth processes occurring at early embryogenesis during seed development, participate in the mechanisms underlying radicle protrusion during seed germination and seed aging.11,12 In plant cells, the most important reducing substrate for H2O2 removal is ascorbic acid which acts as an antioxidant.13 Recently, we have reported that ascorbic acid suppresses the germination of wheat seeds.14 Ascorbate peroxidase (APX) and catalase (CAT) scavenging H2O2 are localized at the site of H2O2 generation in plant cells.15 De Gara et al.16 have followed the changes in detoxifying enzyme activities during the maturation of Triticum durum kernels. Their results show that ascorbic acid, APX and CAT are contained in maturing wheat seeds, whereas the seeds at the end of their development do not contain either ascorbate or APX but do still contain CAT. Seed filling is associated with the high potential of the H2O2 detoxification machinery, mainly due to APX and CAT activities.
In the present study, we examined the role of antioxidant in regulating germination during wheat seed maturation. The aims of the present work were (1) to study the influence of endogenous and exogenous ascorbate in germination during wheat seed maturation, and (2) to understand in more detail the relationship between H2O2 scavenging efficiency and germination ability of developing and maturing wheat seeds.
Results
Germination ability during seed maturation of the two cultivars.
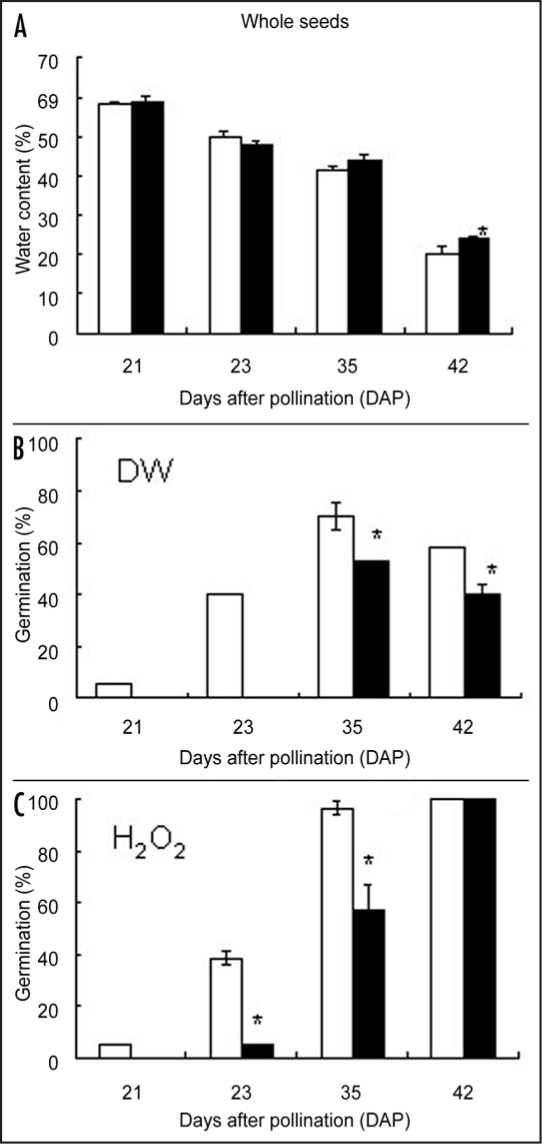
The water contents in the wheat seeds of both cultivars were the highest at 14 DAP and decreased gradually until 42 DAP (Fig. 1A). From 3 to 35 DAP, water content did not differ significantly between the two cultivars. According to the classification of wheat seed development,20 these seeds appear to have reached harvest-maturity at 40 DAP.
Figure 1.
Water content (A), germination ratio of wheat seeds treated by DW (B) (control) and H2O2 (C) in Shirogane-Komugi and Norin61 cultivars during the development and maturation processes. White bars indicate Shirogane-Komugi and black bars indicate Norin61. The reported values are the means and S. D. of five replications. An asterisk indicates statistical significance at the 5% level (Student's t-test).
The germination rate of whole seeds in the two cultivars during maturation is shown in Figure 1B. At 28 DAP, none of the seeds of Norin61 germinated, while aproximately 40% of Shirogane-Komugi germinated. In both cultivars, the highest germination rate was observed at 35 DAP. The germination rates of Shirogane-Komugi were higher than those of Norin61 from 21 to 42 DAP. This tendency was closely consistent with results for the past two years (data not shown). In Shirogane-Komugi, although the germination rate of hydrogen peroxide-treated whole seeds was the same as that of DW-treated seeds at 28 DAP, the germination rate of H2O2-treated whole seeds at 35 and 42 DAPs was almost 100% compared with about 65% for DW-treated whole seeds (Fig. 1B and C). On the other hand, H2O2-treated whole seeds in Norin61 had germinated at the same rate as DW-treated whole seeds at 35 DAP and the ratio was about two times that of DW-treated whole seeds at 42 DAP. These results indicated that H2O2 promoted germination during wheat seed maturation.
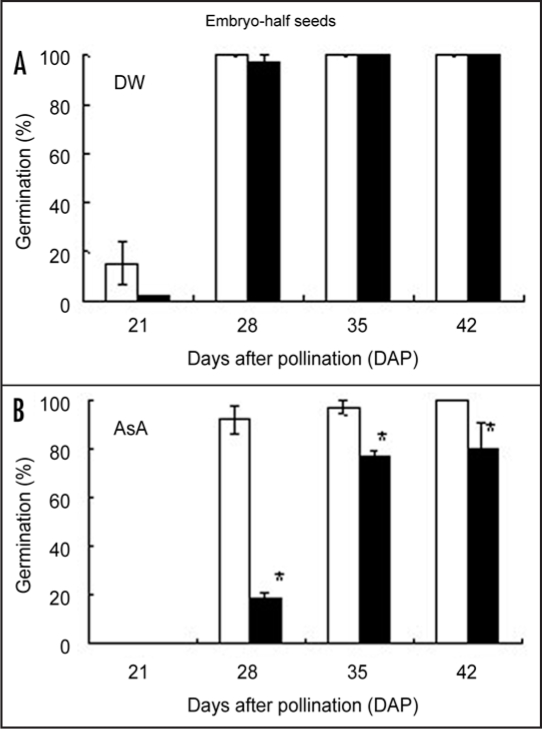
Figure 2.
Sensitivity of ascorbic acid in Shirogane-Komugi and Norin61 embryo-half seeds during development and maturation processes. (A) DW (control), (B) AsA (ascorbic acid). White bars indicate Shirogane-Komugi and black bars indicate Norin61. The reported values are the means and S. D. of five replications. An asterisk indicates statistical significance at the 5% level (Student's t-test).
Ascorbic acid sensitivity during seed maturation of two cultivars.
The germination rate of the embryo-half seeds of two cultivars is shown in Figure 2. Kawakami et al.21 measured ABA sensitivity during the seed maturation of wheat mutants by using embryo-half grains. In both cultivars, the most of DW-treated embryo-half seeds had germinated at 21 DAP. Although the most of Shirogane-Komugi embryo-half seeds treated by AsA germinated, the germination rate of Norin61 was 18% at 28 DAP and was about 80% at 35 and 42 DAPs. Ascorbic acid suppressed specifically Norin61 during the seed maturation. Especially, at 28 DAP, germination of Norin61 embryo-half seeds treated by AsA was suppressed by about 80% as compared with DW-treated embryo-half seeds, while that of Shirogane-Komugi was only negligibly suppressed.
Hydrogen peroxide and ascorbate contents during seed maturation of two cultivars.
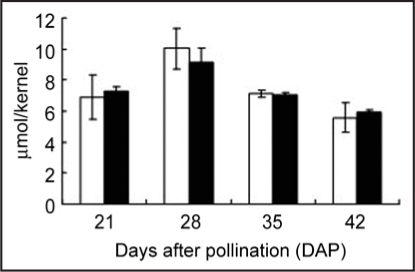
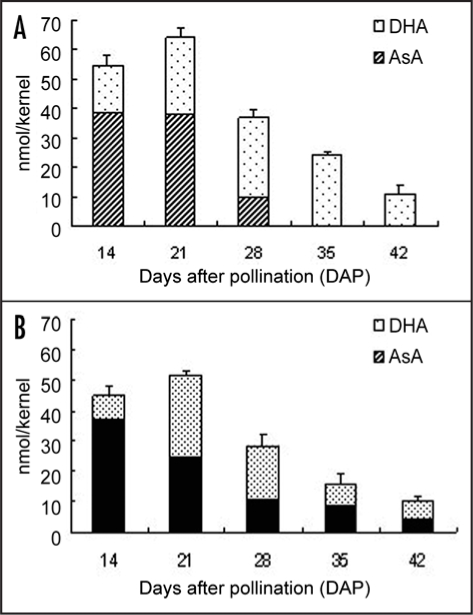
The H2O2 contents in the wheat seeds of both cultivars were the highest at 28 DAP and decreased gradually until 42 DAP (Fig. 3). The H2O2 contents during the seed maturation did not differ significantly between the two cultivars. The total ascorbate content (AsA + DHA) in the whole seeds of both cultivars was the highest at 21 DAP (Fig. 4). In Shirogane-Komugi, ascorbic acid content was high until 21 DAP, markedly decreasing at 28 DAP and then disappearing. On the other hand, the ascorbate contents of Norin61 was highest at 14 DAP, decreased markedly at 28 DAP, and then remained at a constant value until 42 DAP. Although AsA content in Shirogane-Komugi at 21 DAP was higher than that of Norin61, AsA content in Shirogane-Komugi and Norin61 at 28 DAP were 9.8 ± 0.31 and 10.7 ± 3.34 nmol/kernel, respectively, and AsA/DHA ratio in Sirogane-Komugi and Norin61 at 28 DAP were 0.41 ± 0.05 and 0.61 ± 0.17, respectively. AsA content and AsA/DHA ratio at 28 DAP were indicated no significant difference in the two cultivars.
Figure 3.
Contents of hydrogen peroxide in Shirogane-Komugi and Norin61 seeds during development and maturation processes. White bars indicate Shirogane-Komugi and black bars indicate Norin61. The reported values are the means and S.D. of five replications.
Figure 4.
Contents of ascorbate and dehydroascorbate in Shirogane-Komugi and Norin61 seeds during development and maturation processes. (A) Shirogane-Komugi, (B) Norin 61. The reported values are the means and S.D. of five replications.
Activity of antioxidant enzymes and CAT gene expression during seed maturation.
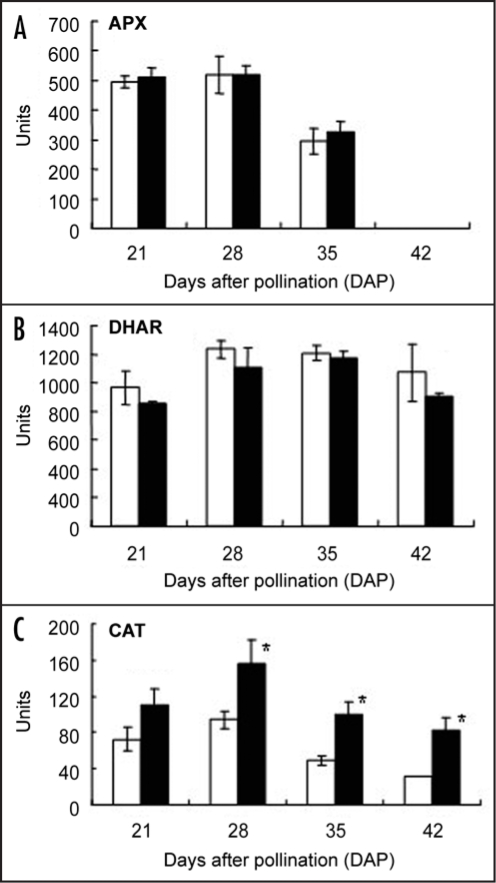
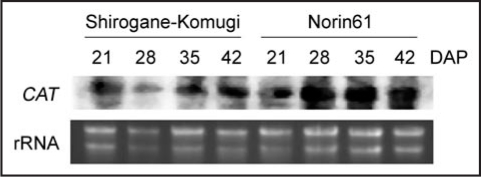
In both cultivars, when determined on a per kernel basis, no significant differences were detected for APX during the first 35 DAP, and the H2O2 scavenging enzyme markedly decreased until it was no longer detectable in the mature kernels (Fig. 5A). DHAR activity acts as AsA recycling enzyme and the activity of DHAR in Shirogane-Komugi increased from 21 to 28 DAP, remained constant for the following week, and then decreased to values similar to that of 21 DAP while that in Norin61 increased at 35 DAP and markedly decreased at 42 DAP (Fig. 5B). CAT activity which has global H2O2 removal capability of kernel cells during maturation was also measured (Fig. 5C). CAT activity in both cultivars during maturation was the highest at 28 DAP, and then gradually decreased. However, CAT activity in Norin61 during maturation was significantly higher than that in Shirogane-Komugi throughout seed maturation. Unlike APX, CAT activity was still present in the dehydrated kernel, and there was significant varietal difference in two cultivars. Based on these results, CAT gene expression during seed maturation was also measured (Fig. 6). CAT gene expression was also remarkably high at 28 DAP in Norin61. This result clearly revealed a similar tendency in CAT activity and its gene expression.
Figure 5.
Antioxidant enzymes in Shirogane-Komugi and Norin61 seeds during development and maturation processes. (A) APX (ascorbate peroxidase), (B) DHAR (dehydroascobate reductase), (C) CAT (catalase). White bars indicate Shirogane-Komugi and black bars indicate Norin61. The reported values are the means and S.D. of five replications. 1 unit = 1 nmol ascorbate oxidized min-1 kernel-1 (APX); 1 nmol DHA reduced min-1 kernel-1 (DHAR); 1 nmol H2O2 dismutated min-1 kernel-1. An asterisk indicates statistical significance at the 5% level (Student's t-test).
Figure 6.
Northern blots of CAT gene expression in Shirogane-Komugi and Norin61 seeds during development and maturation processes.
Discussion
Water contents during maturation did not show a significant difference between the two cultivars (Fig. 1A). However, the seeds of Norin61 were significantly more germination ability than those of Shirogane-Komugi from 21 to 42 DAP (Fig. 1B). These results indicated that the two cultivars have a physiological difference in the germination characteristics during seed maturation.
H2O2 enhanced the germination rate in wheat, rice, and barley22 and is produced at the early imbibition period in wheat,23 soybean,24 radish,25 maize,26 sunflower,27 and tomato28 seeds. Indeed, in the present study, H2O2 enhanced the germination rate and the influence of this promotion was significantly different during maturation in wheat cultivars (Fig. 1C). Phenolic compounds which act as an antioxidant suppressed seed germination.10 Recently, we have reported that ascorbic acid acts as an antioxidant scavenging H2O2 and suppresses the germination of mature wheat seeds.14 Therefore, we examined sensitivity to ascorbic acid in developing and maturing seeds of wheat cultivars having different germination characteristics (Fig. 2).
ROS generation is known to occur during the dehydration of various plant tissues.29 In wheat leaves, drought-induced H2O2 accumulation correlated with a decrease in soil water content, and leaf H2O2 content increased even though CAT activity doubled under severe drought conditions.30 It has also been demonstrated that the desiccation of developing sunflower seeds is associated with an increase in CAT activity.31 In the present study, water contents of seeds in the two cultivars during maturation did not show significant difference (Fig. 1A), and H2O2 content of the seeds had no significant difference in the two cultivars (Fig. 3). On the other hand, sensitivity to AsA of developing seeds was higher in Norin61 than in Shirogane-Komugi. Additionally, the sensitivity to AsA was closely associated with germination characteristic in the two cultivars. This poses the question as to why significant differences exist in wheat cultivars affected by ascorbic acid.
De Tullio and Arrigoni32 showed that the ascorbic acid system functions dynamically in seeds. The strategies for ascorbic acid production and utilization may vary according to developmental and functional stages in seeds, while ascorbic acid function in seeds is also likely to be related to its action as a specific cosubstrate required for the activity of dioxygenases involved in the synthesis of ethylene, gibberellins, and abscisic acid, respectively. Hydrogen peroxide is eliminated by ascorbate peroxidase (APX) and catalase (CAT).33,34 These enzymes rapidly destroy the vast majority of H2O2 produced by metabolism.13 We inferred that the difference of ascorbic acid sensitivity in the two cultivars is caused by a difference in the amount of endogenous ascorbate or a difference of APX or CAT activity.
Ascorbate contents at 28 DAP was not significantly different in the two cultivars, while ascorbate contents at 35 and 42 DAP in Norin61 were higher than in Shirogane-Komugi (Fig. 4). These results indicated that AsA synthesis ability at 35 and 42 DAPs in Norin61 were higher than in Shirogane-Komugi because there were no differences in endogenous H2O2 content, APX and DHAR activities (Figs. 3, 5A and B) and the difference of sensitivity to ascorbic acid at 35 and 42 DAPs was contributed by endogenous ascorbic acid in seeds. However, the difference of sensitivity to AsA at 28 DAP could not prove by endogenous AsA only. It has been widely reported that APX plays a key role in removing the toxic H2O2 in plant cells,35,36,37 the correct temporal expression of APX has to be an important factor for seed germination.38 APX activity in the two cultivar seeds disappeared at the end of their development and did not show any significant differences during seed maturation (Fig. 5A). In addition, DHAR activity was also not significantly different in the two wheat cultivars (Fig. 5B). CAT performs important roles in seed germination. In sunflower seeds, a quite close relationship between CAT activity and germination rate has (previously reported in ref. 39). Maize CAT genes have response elements that allow for fine tuning of their expression by ABA and GA.40,41 CAT activity in Norin61 was higher than that in Shirogane-Komugi during seed maturation (Fig. 5C). Especially, at 28 DAP, CAT activity of Norin61 seed was remarkably high. In addition, the mRNA level of the CAT gene was also highest from 28 to 42 DAP in Norin61 (Fig. 6). These results indicated that the difference of sensitivity to ascorbic acid at 28 DAP was closely contributed by CAT activity in seeds. In the present study, it was indicated that the difference of ascorbic acid sensitivity in the two cultivars was caused by a difference in endogenous ascorbate content, CAT activity, and its gene expression. Namely, sensitivity to AsA in Norin61 was higher than that of Shirogane-Komugi because H2O2 scavenging ability was high in Norin61 (Figs. 2–6). This indicates that germination during seed maturation in wheat seeds is closely related to H2O2 scavenging ability during maturation.
Walker-Simmons42 showed that ABA sensitivity disappeared in parallel with the loss of dormancy during seed development and after ripening, and was a key factor of seed dormancy rather than the ABA content of the wheat embryos. Recently, it was reported interfere with ABA metabolism and seed dormancy in arabidopsis and barley.43 mRNAs encoding superoxide dismutase (SOD), CAT and APX were almost undetectable in aleurone layers 24 h after incubation in gibberellin (GA).44 For aleurone layers incubated in ABA, however, the amounts of these mRNA increase. Western blotting and enzyme activity assays confirm that GA but not ABA reduced the amount and activity of ROS scavenging enzymes. Seed germination and dormancy may be possibly influenced by ROS scavenging ability controlled by ABA metabolism.
In this paper we showed a direct correlation between germination ability and antioxidant sensitivity during the developmental stages of wheat seeds. Therefore, it was indicated that H2O2 scavenging substances and enzymes are related to wheat seed germination ability during maturation, and H2O2 scavenging was one of the important factors related to premature germination of seeds.
Materials and Methods
Plant growth.
Common wheat (Triticum aestivum L.) cultivars, Shirogane-Komugi and Norin61, were grown in the experimental fields of Kyushu University at Fukuoka in 2004–2005 on 30m2 plots. Ear flowering started on 14 April 2005 and the two cultivars were protected against rain under a transparent vinyl roof. Irrigation, fertilization, and plant protection were performed to ensure optimal plant growth. The seeds were sampled at the primary and secondary florets of the central spikelets at 7 d intervals after anthesis and stored at -80°C for analysis.
Germination was determined in five replications of twenty fresh seeds or half seeds containing embryos (embryo-half seeds) incubated in a Petri dish (9 cm in diameter) containing filter paper moistened with 6 ml of distilled water (DW), 10 mM H2O2, and 50 mM aqueous solution of ascorbic acid at 15°C for 7 d in a dark condition.
Water contents.
Dry seed weight was measured after the incubation of 20 seeds dried at 90°C for 20 h. Water content (%) of seeds was calculated in terms of (fresh weight - dry weight) / fresh weight.
Analysis of ascorbate and hydrogen peroxide.
The seeds (1 g) were homogenized with 8 ml of 5% (w/v) meta-phosphoric acid in liquid N2. The homogenate was centrifuged at 20000 g for 15 min at 4°C, and the supernatant was used for analysis of ascorbate contents. The AsA and DHA content were determined according to the method (described in ref. 17). The H2O2 content was measured colorimetrically (described in ref. 18). H2O2 was extracted by homogenizing wheat seeds with phosphate buffer (50 mM, pH 6.5) containing 1 mM hydroxylamine. The homogenate was centrifuged at 6000 g for 25min. To determine H2O2 content, the extracted solution was mixed with 0.1% titanium sulphate in 20% (v/v) H2SO4. The mixture was then centrifuged at 6000 g for 25 min. The absorbance was measured at 410 nm.
Enzyme activity.
The seeds (1 g) were ground in liquid N2 and then resuspended in 4ml of a medium [50 mM Tris-HCl (pH 7.8), 0.05% (w/v) cysteine, and 0.1 % (w/v) BSA], just as the last trace of liquid N2 disappeared. The thawed homogenate was then ground and centrifuged at 20000 g for 15 min at 4°C. The supernatant was used for spectrophotometric analysis.
Ascorbate peroxidase (L-ascorbate: hydrogen peroxide oxido-reductase, EC 1.11.1.11) and DHA reductase (glutathione: dehydroascorbate oxidoreductase, EC 1.8.5.1) were assayed according to the method (described in ref. 19).
Catalase (hydrogen peroxide: hydrogen peroxide oxidoreductase, EC 1.11.1.6) activity assay was performed according to the method described by De Gara et al. by monitoring the decrease of ABS240nm of the reaction mixture as H2O2 dismutation, which consisted of 0.1 M phosphate buffer, pH 7.0, 50–100 µg protein, and 18 mM H2O2 (extinction coefficient 23.5 mM-1 cm-1). Enzyme activities were calculated on a per-seed basis.
Northern blot analysis.
Total RNA was isolated from seeds during maturation (21 DAP to 42 DAP) using the sodium dodecyl sulfate (SDS)-phenol lithium chloride method. PCR products of wheat CAT ORF (NCBI accession no. D86327) were amplified from cDNA, and digoxigenin (DIG) labelling of the PCR products was carried out using a nonisotopic DIG-labelling kit (Roche Diagnostic). Total RNA (1 µg) was denatured in a mixture of 70% (v/v) formamide and 8% (v/v) formaldehyde, and separated on a 1.5% agarose gel containing 1.8% (v/v) formaldehyde. After electrophoresis, RNAs were transferred to a Hybond-N+ membrane (Amersham Biosciences). Prehybridization of the membrane was carried out at 65°C for 1 h in 0.3 M phosphate buffer containing 7% SDS, and hybridization was then carried out by incubating the membrane in the same buffer with DIG-labelled probes at 65°C for over 15 h. Membranes were washed in 2x SSC containing 0.1% SDS (15 min), and then in 0.1x SSC containing 0.1% SDS (15 min) at 65°C. After incubation of the blots with anti-DIG antibody-horse-radish peroxidase at 37°C for 1 h, DIG-epitopes on membranes were detected by Fluorchem (Alpha Innotech) using an ECL kit (Amersham Biosciences).
Acknowledgements
This work was supported by research fellowships from the JSPS.
Abbreviations
- ABS
absorbance
- AsA
ascorbic acid
- APX
ascorbate peroxidase
- CAT
catalase
- DAP
days after pollination
- DHA
dehydro-ascorbate
- DHAR
dehydroascorbate reductase
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/5540
References
- 1.Koornneef M, Bentsink L, Hilhorst H. Seed dormancy and germination. Curr Opin Plant Biol. 2002;5:33–36. doi: 10.1016/s1369-5266(01)00219-9. [DOI] [PubMed] [Google Scholar]
- 2.Bewley JD. Seed germination and dormancy. Plant Cell. 1997;9:1055–1066. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gfeller F, Svejda F. Inheritance of post-harvest seed dormancy and kernel colour in spring wheat lines. Can J Plant Sci. 1960;40:1–6. [Google Scholar]
- 4.Mares DJ. Mechanism and genetic control of dormancy in wheat. In: Lang GA, editor. 1st International Symposium on Plant Dormancy; Corvallis. 1994. p. 39. (symposium abstract) [Google Scholar]
- 5.Himi E, Mares DJ, Yanagisawa A, Noda K. Effect of grain colour gene (R) on grain dormancy and sensitivity of the embryo to abscisic acid (ABA) in wheat. J Exp Bot. 2002;53:1569–1574. doi: 10.1093/jxb/erf005. [DOI] [PubMed] [Google Scholar]
- 6.Weidner S, Paprocka J. Preharvest sprouting as related to change in concentration of phenolic compounds in cereal grain and embryo sensitivity to phenolic acids during seed development; COST 828 Workgroup Meeting; Barcelona. 1997. p. 2. [Google Scholar]
- 7.McDonald MB. Seed deterioration: Physiology repair and assessment. Seed Sci Technol. 1999;27:177–237. [Google Scholar]
- 8.Fontaine O, Huault C, Pavis N, Billard JP. Dormancy breakage of Hordeum vulgare seeds: Effect of hydrogen peroxide and scarification on glutathione level and glutathione reductase activity. Plant Physiol Biochem. 1994;32:677–683. [Google Scholar]
- 9.Chien CT, Lin TP. Mechanism of hydrogen peroxide in improving the germination of Cinnamonum camphora seed. J Exp Bot. 1994;44:127–132. [Google Scholar]
- 10.Ogawa K, Iwabuchi M. A mechanism for promoting the germination of Zinia elegans seeds by hydrogen peroxide. Plant Cell Physiol. 2001;42:286–291. doi: 10.1093/pcp/pce032. [DOI] [PubMed] [Google Scholar]
- 11.Bailly C. Active oxygen species and antioxidants in seed biology. Seed Sci Res. 2004;14:93–107. [Google Scholar]
- 12.Kibinza S, Vinel D, Côme D, Bailly C, Corbineau F. Sunflower seed deterioration as related to moisture content during ageing, energy metabolism and active oxygen species scavenging. Physiol Plant. 2006;128:496–506. [Google Scholar]
- 13.Noctor G, Foyer CH. Ascorbate and glutathione: Keeping active oxygen under control. Annu Rev Plant Physiol Mol Biol. 1998;49:729–737. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- 14.Ishibashi Y, Iwaya-Inoue M. Ascorbic acid suppresses germination and dynamic states of water in wheat seeds. Plant Prod Sci. 2006;9:172–175. [Google Scholar]
- 15.Asada K. The water-water cycle in chloroplasts: Scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Mol Biol. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- 16.De Gara L, de Pinto MC, Moliterni VMC, D'Egidio MG. Redox reguraion and storage processes during maturation in kernels of Triticum durum. J Exp Bot. 2003;54:249–258. doi: 10.1093/jxb/erg021. [DOI] [PubMed] [Google Scholar]
- 17.Kampfenkel K, Van Montagu M, Inzé D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem. 1995;225:165–167. doi: 10.1006/abio.1995.1127. [DOI] [PubMed] [Google Scholar]
- 18.Hung KT, Kao CH. Hydrogen peroxide is necessary for abscisic acid induced senescence of rice leaves. J Plant Physiol. 2004;161:1347–1357. doi: 10.1016/j.jplph.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Di Cagno R, Guidi L, De Gara L, Soldatini G. Combined cadmium and ozone treatments affect photosynthesis and ascorbate-dependent defenses in sunflower. New Phytol. 2001;151:627–636. doi: 10.1046/j.1469-8137.2001.00217.x. [DOI] [PubMed] [Google Scholar]
- 20.Rogers SO, Quatrano RS. Morphological staging of wheat caryopsis development. Am J Bot. 1983;70:308–311. [Google Scholar]
- 21.Kawakami N, Miyake Y, Noda K. ABA insensitivity and low ABA levels during grain development of nondormant wheat mutants. J Exp Bot. 1997;48:1415–1421. [Google Scholar]
- 22.Neredo MEB, Juliano AB, Lu BR, de Guzman F, Jackson MT. Responses to seed dormancy-braking treatments in rice species (Oryza L.) Seed Sci Technol. 1998;26:675–689. [Google Scholar]
- 23.Caliskan M, Cumming AC. Spatial specificity of H2O2-generating oxalate oxidase gene expression during wheat embryo germination. Plant J. 1998;15:165–171. doi: 10.1046/j.1365-313x.1998.00191.x. [DOI] [PubMed] [Google Scholar]
- 24.Puntarulo S, Sanchez RA, Boveris A. Hydrogen peroxide metabolism in soybean embryonic axes at the onset of germination. Plant Physiol. 1988;86:626–630. doi: 10.1104/pp.86.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schopfer P, Plachy C, Frahry G. Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiol. 2001;125:1591–1602. doi: 10.1104/pp.125.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hite DRC, Auh C, Scandalios JG. Catalase activity and hydrogen peroxide levels are inversely correlated in maize scutella during seed germination. Red Rep. 1999;4:29–34. doi: 10.1179/135100099101534710. [DOI] [PubMed] [Google Scholar]
- 27.Bailly C, Bogatek-Leszczynska R, Côme D, Corbineau F. Changes in activities of antioxidant enzymes and lipoxygenase during growth of sunflower seedlings from seeds of different vigour. Seed Sci Res. 2002;12:47–55. [Google Scholar]
- 28.Morohashi Y. Peroxidase activity develops in the micropylar endosperm of tomato seeds prior to radicle protrusion. J Exp Bot. 2002;53:1643–1650. doi: 10.1093/jxb/erf012. [DOI] [PubMed] [Google Scholar]
- 29.Smirnoff N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993;125:27–58. doi: 10.1111/j.1469-8137.1993.tb03863.x. [DOI] [PubMed] [Google Scholar]
- 30.Luna CM, Pastori GM, Driscoll S, Groten K, Bernard S, Foyer CH. Drought controls on H2O2 accumulation, catalase (CAT) activity and CAT gene expression in wheat. J Exp Bot. 2004;56:417–423. doi: 10.1093/jxb/eri039. [DOI] [PubMed] [Google Scholar]
- 31.Bailly C, Leymarie J, Lehner A, Rousseau S, Côme D, Corbineau F. Catalase activity and expression in developing sunflower seeds as related to drying. J Exp Bot. 2004;55:475–483. doi: 10.1093/jxb/erh050. [DOI] [PubMed] [Google Scholar]
- 32.De Tullio MC, Arrigoni O. The ascorbic acid system in seeds: To protect and to serve. Seed Sci Res. 2003;13:249–260. [Google Scholar]
- 33.Chen GX, Asada K. Ascorbate peroxidase in tea leaves: Occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol. 1989;30:987–998. [Google Scholar]
- 34.Scandalios JG, Guan L, Polidoros AN. Catalases in plants: Gene structure, properties, regulation, and expression. In: Scandalios JG, editor. Oxidative Stress and the Molecular Biology of Antioxidant Defenses. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1997. pp. 343–406. [Google Scholar]
- 35.Mittler R, Zilinskas BA. Molecular cloning and nucleotide sequence analysis of a cDNA encoding pea cytosolic ascormate peroxidase. FEBS lett. 1991;289:257–259. doi: 10.1016/0014-5793(91)81083-k. [DOI] [PubMed] [Google Scholar]
- 36.Asada K. Ascorbate peoxidase a hydrogen peroxide-scavenging enzyme in plants. Physiol Plant. 1992;85:235–241. [Google Scholar]
- 37.Yamaguchi K, Mori H, Nishimura M. A novel isoenzyme of ascorbate peroxidase localized on glyoxisomal and leaf peroxisomal membrane in pumpkin. Plant Cell Physiol. 1995;36:1157–1162. doi: 10.1093/oxfordjournals.pcp.a078862. [DOI] [PubMed] [Google Scholar]
- 38.De Gara L, de Pinto MC, Arrigoni O. Ascorbate synthesis and ascorbate peoxidase activity during the early stage of wheat germination. Physiol Plant. 1997;100:894–900. [Google Scholar]
- 39.Bailly C, Benamar A, Corbineau F, Côme D. Free radical scavenging as affected by accelerated ageing and subsequent priming in sunflower seeds. Physiol Plant. 1998;104:646–652. [Google Scholar]
- 40.Guan LM, Sacandalios JG. Molecular evolution of maize catalases and their relationship to other eukaryotic and prokaryotic catalases. J Mol Evol. 1996;42:570–579. doi: 10.1007/BF02352287. [DOI] [PubMed] [Google Scholar]
- 41.Guan LM, Zhao J, Sacandalios JG. Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signalling molecule for the response. Plant J. 2000;22:87–95. doi: 10.1046/j.1365-313x.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- 42.Walker-Simmons M. ABA levels and sensitivity in developing wheat embryos of sprouting resistant and susceptible cultivars. Plant Physiol. 1987;84:61–66. doi: 10.1104/pp.84.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Millar AA, Jacobsen JV, Ross JJ, Helliwell CA, Poole AT, Scofield G, Reid JB, Gubler F. Seed dormancy and ABA metabolism in Arabidopsis and barley: The role of ABA 8'-hydroxylase. Plant J. 2006;45:942–956. doi: 10.1111/j.1365-313X.2006.02659.x. [DOI] [PubMed] [Google Scholar]
- 44.Fath A, Bethke P, Beligni V, Jones R. Active Oxygen and cell death in cereal aleurone cells. J Exp Bot. 2002;53:1273–1283. [PubMed] [Google Scholar]