Abstract
In plant/parasitic plant interaction, little is known about the host plant response before the establishment of the parasite within the host. In the present work, we focused on host responses to parasitic plant, O. ramosa in the early stage of infection. We used a co-culture system of A. thaliana suspension cells and O. ramosa germinated-seeds to avoid parasite attachment. We showed that O. ramosa induced H2O2 generation and camalexin synthesis by A. thaliana followed by a drastic increase in cell death. We further demonstrated that a heat sensitive diffusible signal is responsible for this cell death. These data indicate that recognition of O. ramosa occurs before the attachment of the parasite and initiates plant defence responses.
Key words: Orobanche ramosa, Arabidopsis thaliana, cell death, hydrogen peroxide, secondary metabolism
Introduction
Parasitic plants cause severe yield losses, especially the parasitic plants belonging to the Scrophulariaceae and Orobanchaceae families.1 Some Orobanche species have wide host ranges and grow on annual hosts.2 These species are able to parasite a wide range of agricultural crops1 and cause major yield losses.1,3,4 O. ramosa is one of the most noxious especially in Europe but damages were also reported in Australia and Chile. In France, this parasite causes heavy losses in hemp, oilseed rape, tobacco and tomato.5 Orobanche sp. is a root holoparasite completely dependent on the host plant for nutrients and water. It attaches and invades the host roots using a specialized structure, called haustorium. Parasite germination and host attachment are supposed to be the most critical steps required for the accomplishment of the parasite life cycle. They are dependent on different host plant molecules, sesquiterpenes or hydroquinones and benzoquinones.6–9 These molecules are responsible for parasite germination and haustoria formation, respectively. On the other hand, the host perception of parasitic plants in this early stage of the interaction could be important as in numerous plant/pathogen interactions. In plant/pathogenic microorganism interactions, several pathogen-derived key molecules have been isolated. They are called elicitors because they induce plant defense responses. The general plant responses against pathogens are programmed cell death, oxidative burst, phytoalexin production and accumulation of antimicrobial proteins such as pathogenesis related (PR) proteins. In the last few years, some studies on plant responses to parasitic plants revealed that parasite attachment to the host root also promote the plant defense responses which observed in plant/pathogen interaction. Indeed, PR-1 gene promoter is activated in transformed tobacco roots after the attachment of Orobanche aegyptica.10 Hmg2, a related defense gene in tomato is also activated after root penetration by O. aegyptica.11 NRSA-1 encoding a putative disease resistance protein is induced in marigold roots parasitized by Striga spp.12 The enhanced-expression of LeAqp2, an aquaporin-like gene, was reported in Lycopersicum esculentum in response to Cuscuta reflexa.13 More recently, a proteomic study on pea responses to O. crenata reported the upregulation of PR proteins including β-1,3-glucanase and peroxidase.14 Vieira Dos Santos et al.,15,16 also showed the activation of A. thaliana defense genes in response to O. ramosa such as those coding for defensin (pdf1.2), PR-3, thionin (thi2.1), pectin methyl esterase inhibitor (PMEi), reactive oxygen species (ROS) detoxifying proteins, gst1, gst11 and peptide methionine sulfoxide reductase. These authors detected A. thaliana gene activation before the parasite attachment by the use of an in vitro co-culture system with A. thaliana callus.16 This is consistent with the hypothesis that A. thaliana perceive the presence of O. ramosa without parasitic attachment. Although these studies indicate that parasitism promote the expression of defense related genes in host, the early plant defense responses against O. ramosa are still obscure. To clarify the early perception-based responses, we chose A. thaliana as the host of the parasitic plant O. ramosa, the most damaging species of dicotyledonous species in France.5,17 An in vitro co-culture system using A. thaliana suspension cells allowed us to study the first stage of interaction preceding the parasitic attachment. In the last decade it has been demonstrated that use of suspension cultured cells is convenient for identifying early physiological events induced by pathogens.18–22 Therefore, here we attempted to examine (i) the detection of cell death induction in A. thaliana cells by O. ramosa, which is known to occur both in compatible or incompatible host-parasite interactions, (ii) the quantification of oxidative burst represented by production of ROS, especially H2O2, which is one of the most commonly reported plant responses to biotic stresses,23 and (iii) the evaluation of phytoalexin production.
Results
In this study, we used a co-culture system with O. ramosa seeds and A. thaliana cells to examine the induction of physiological defense related responses in A. thaliana (Fig. 1) independently of any physical attachment. Because the O. ramosa seeds remain inactive in absence of host root exudates,6–9 they were treated, before co-culture, with a synthetic analog of strigol,24 which allows their germination in absence of such exudates.
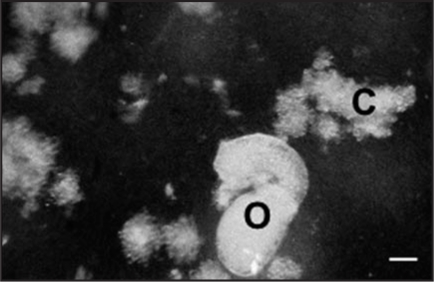
Figure 1.
Five days co-culture of A. thaliana suspension cells (C) and O. ramosa germinated seeds (O). Bar = 280 µm.
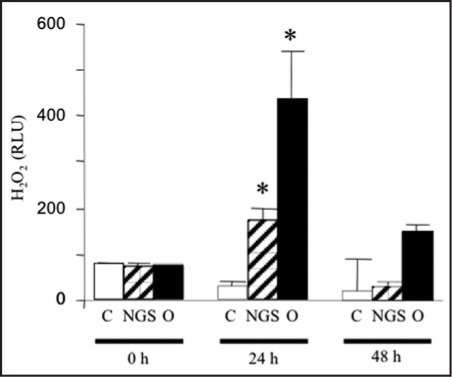
The production of ROS is one of the most common events related to cell death in plant-pathogen interactions. Thus, we tested the putative effect of germinating O. ramosa seeds on ROS production. A significant increase in luminol-mediated chemioluminescence caused by H2O2 release in the culture medium of A. thaliana cells infected by O. ramosa seeds was observed. This increase in H2O2 production occurred during the first day of interaction and then decreased consistent with an oxidative burst feature (Fig. 2). The control without seeds of O. ramosa did not show any significant increase in H2O2 production throughout the experiments. Non-germinated seeds also showed increase in H2O2 production but only to a lesser extent.
Figure 2.
Effect of O. ramosa on production of H2O2 by A. thaliana cell suspensions. Accumulation of H2O2 in the medium of A. thaliana cell suspensions at 0, 24 and 48 hours of presence of O. ramosa germinated seeds (O), non-germinated seeds (NGS) and without seeds (C). Data were obtained from six independent experiments. *significantly different from the control (p < 0.05).
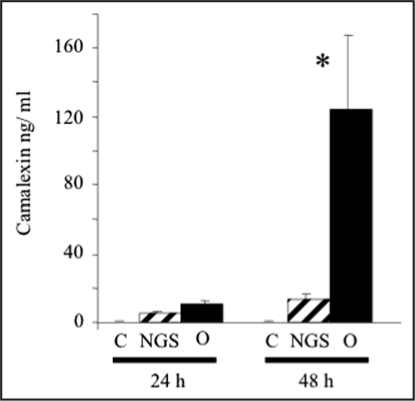
O. ramosa germinated seeds also induced synthesis of camalexin, the main A. thaliana phytoalexin.31 Even after 24 h of co-incubation, no significant change in camalexin level could be detected. After 48 h, germinating seeds induced a significant level of camalexin synthesis (Fig. 3).
Figure 3.
Effect of O. ramosa on camalexin production in A. thaliana suspension cells. Camalexin synthesis in response to 24 and 48 h of presence of O. ramosa germinated seeds (O), non germinated seeds (NGS) and without seeds (C). Data are mean of four independent samples. * significantly different from the control (p < 0.05).
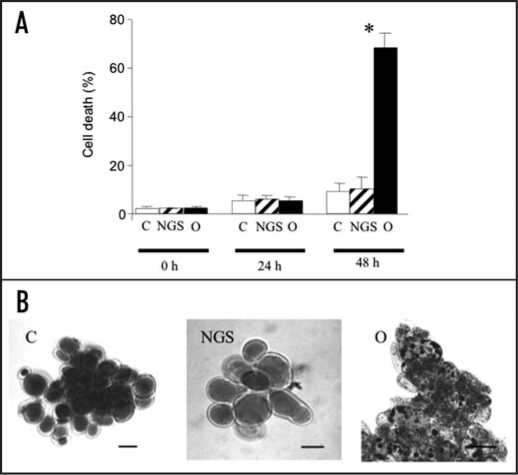
Furthermore, we showed that O. ramosa germinated seeds induced death of A. thaliana suspension cells. Quantification of cell death was made with Evans blue staining. After 24 h, no significant variation of cell death could be observed between the controls and the suspension cells infected by germinated O. ramosa seeds. After 48 h, germinating seeds induced a large death of A. thaliana cells (Fig. 4A). Vital staining with neutral red further revealed that this cell death was accompanied with an important condensation and a vacuolization of the cell cytoplasm (Fig. 4B).
Figure 4.
O. ramosa induced cell death in A. thaliana suspension cells. (A) Effect of O. ramosa germinated (O) and non-germinated seeds (NGS) on A. thaliana cell death evaluated with Blue Evans staining after 0, 24 and 48 h of co-culture. C corresponds to the control without O. ramosa seeds. Data were obtained from 10 independent experiments and error bars correspond to standard errors. *significantly different from the control (p < 0.05). (B) Neutral red staining of A. thaliana cells after 48 hours of the same treatments. Bar = 40 µm.
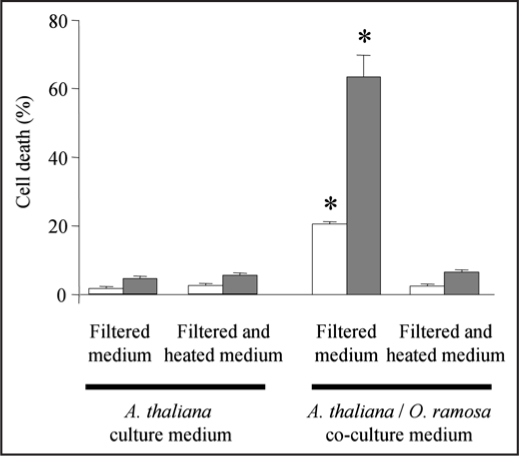
To assess the putative involvement of a diffusible signal secreted by the germinated seeds from O. ramosa, we tested the effect of a 12 or 24 h germinated O. ramosa/A. thaliana filtered co-cultured medium on A. thaliana cell death. Both filtered mediums were found to induce cell death after 24 h but at different extent (Fig. 5). These data indicate that a diffusible signal released by germinated O. ramosa seeds during co-culture could elicit cell death in absence of the seeds. The largest cell death was observed with the medium collected after 24 h of co-culture (Fig. 5) further suggesting that the quantity of a diffusible signal increased with the duration of co-culture. A heat treatment of this medium (5 min at 100°C) completely inhibited the A. thaliana cell death induction indicating that the diffusible signal is heat sensitive (Fig. 5).
Figure 5.
A diffusible signal from O. ramose seeds induced cell death in A. thaliana suspension cells. Co-culture mediums of O. ramosa germinated seeds and A. thaliana cells were filtered and heated, or not, after 12 h (white bars) or 24 h (grey bars) of co-culture. The effect of these mediums were assayed on cell death from fresh culture of A. thaliana cells with Blue Evans staining after 24 h of treatment. Culture medium of A. thaliana cells without O. ramosa was treated in the same way and used as a control. Data were obtained from 4 independent experiments and error bars correspond to standard errors. *significantly different from the control (p < 0.05).
Discussion
In this work, we used a sterile in vitro co-culture system of A. thaliana suspension cells and O. ramosa seeds to study the host responses prior host/parasite contact. We show for the first time that O. ramosa could induce host cell defense responses independently of any contact. O. ramosa stimulated secondary metabolism pathways leading to the synthesis of the phytolalexin, camalexin (Fig. 3). O. ramosa also induced an oxidative burst revealed by the generation of hydrogen peroxide (H2O2) in the culture medium (Fig. 2). The oxidative burst represented by production of reactive oxygen species (ROS) is one of the most common events related to pathogen-plant interaction. The generation of H2O2 in the culture medium precedes the induction of camalexin (Fig. 3) and could thus participate to the pathway leading to this induction. Several studies have effectively demonstrated that H2O2 modulates the gene expression and signaling pathways during defense responses.32,33 The generation of H2O2 also precedes the induction of A. thaliana cell death (Fig. 3). In the same way, H2O2 could be a cell death-inducing signal in the plant/parasitic plant interaction. In fact, H2O2 increased following elicitor treatment or pathogen challenge34,35 effectively acts as a signal triggering the cell death and systemic defense responses.28,36 We show for the first time that O. ramosa could induce host cell death before the physical contact (Figs. 4 and 5). The dead cells present a condensation and a vacuolization of the cytoplasm (Fig. 4), which are morphological events that accompany programmed cell death during hypersensitive response in plants and apoptosis in animal.37 This type of cell death appeared thus clearly different of the one observed during the crush of host cells in the parasite fixation site resulting from a mechanical pressure.38–40 The existence of an induced cell death before parasitic plant contact could act in the beginning of establishment in order to facilitate its penetration into plant tissue. Such behavior was reported by Govrin and Levine41 with the necrotrophic fungi Botrytis cinerea which induced an oxidative burst and a HR cell death in Arabidopsis. Although the aim of a parasitic plant, which could be considered biotrophic, is different of the one of a necrotrophic fungi these both kinds of organisms could exploit a host defense mechanism to facilitate their colonization of host tissue. The cell death we observed with the culture filtrate of germinated O. ramosa seed co-cultured with A. thaliana cells (Fig. 5) indicates that a diffusible signal is secreted by germinated O. ramosa seed. This hypothesis was proposed by Vieira Dos Santos et al.,15 after observing the A. thaliana gene inductions in response to O. ramosa presented without attachment to host roots. Although there is no information about the existence of parasitic plant-derived elicitors analogous to those of microorganisms, the secretion of pectin methyl esterase (PME)42,43 and the evidence of polygalacturonase and cellulase activities44 by various parasitic plants could act as elicitors in addition to their role to open the way for intrusive parasite cells. Secretion of endopolygalacturonase by the germinated seeds could participate directly to cell death induction since this kind of enzyme was recently shown to induce signaling pathway leading to programmed cell death in soybean cells.45 The heat sensitivity of the diffusible signal secreted by germinated O. ramosa seeds (Fig. 5) further suggests a proteinaceous nature for this parasitic plant derived signal.
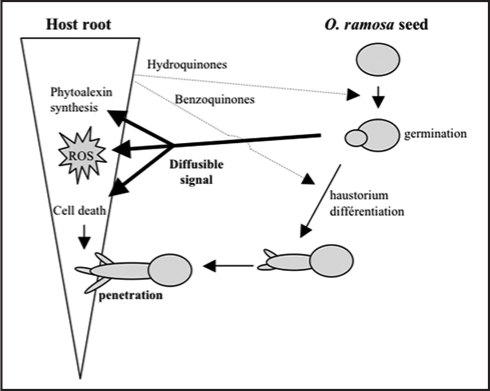
In conclusion, we provide in this study the first evidences supporting the hypothesis that defense responses against O. ramosa germinated seeds are promoted in host plant before the physical contact. We further showed that the activation of A. thaliana physiological and molecular defence responses to O. ramosa is probably induced by a O. ramosa-derived signal molecule. The host plant response induced during this plant-plant interaction (summarized in Fig. 6) shared some cellular events comparable to those induced by invariant pathogen-associated molecular patterns (PAMPs) during plant microbe interactions.46,47 Perception of a PAMPs-like parasitic plant derived signal by host plant cells raises the question of the nonself discrimination between two plants. Further studies are thus need to purify this O. ramosa-derived signal molecule and analyze if host defense responses are governed by a specific recognition between the plant and this pathogen signal or induction of innate immunity.
Figure 6.
Putative model of early interactions during host root and O. ramosa seeds.
Experimental Procedures
Plant material.
O. ramosa L. seeds were collected from an infected winter rape field in Poitou-charente, France. Surface-sterilized seeds were preconditioned in Petri dishes containing glass fiber filter paper moistened with sterile distillated water. Preconditioning was performed in darkness at 20°C for 7 days. O. ramosa germinated seeds were obtained after a treatment with an aqueous solution of GR24 (5 ppm), a synthetic analog of strigol24 which allows the germination in absence of host root exudates. Five days later, GR24 pre-treated seeds were collected, washed five times with sterile water to remove GR24 molecules and used for A. thaliana infestation.
A. thaliana L. (ecotype Columbia) suspension cells were grown at 24 ± 2°C, under continuous white light (40 µE.m-2.s-1) with rotation shaking, in a 1 liter round bottom flask containing 350 ml Gamborg culture medium.25–27 The pH of the culture medium was 5.8. Cells were sub-cultured weekly by a 10-fold dilution. The experiments were conducted on 4-day-old cultures.
A. thaliana cells infestation.
Germinated O. ramosa seeds (30 mg) were placed in round bottom flask containing 25 ml of A. thaliana 4-day old cell cultures. A. thaliana cells and germinated O. ramosa seeds were incubated for various periods. Figure 1 show A. thaliana and O. ramosa after 5 days co-culture. We used non-infected A. thaliana cells (C) and A. thaliana cells incubated with the same amount of non-germinated O. ramosa seeds (non-treated with GR24) (NGS) to escape non specific response.
Experiments with culture filtrate.
Co-culture of A. thaliana cells and germinated O. ramosa were conducted as described before. After 12 or 24 h of co-culture, cells and seeds were removed from the medium by filtration on 50 µm filter in sterile conditions. Four days old A. thaliana cells (1,25 mg) from a new culture were then placed in 25 ml of this medium previously heated (5 min at 100°C) or not.
Cell death detection.
Cell viability was assayed using vital dye, Evans Blue.28 A. thaliana cell cultures (50 µL) were incubated for 5 min in 1 ml phosphate buffer pH 7 supplemented with Evans Blue to a final concentration of 0.005% after 0, 24 and 48 h of treatment. Cells that accumulate Evans blue were considered dying. At least 1000 cells were counted for each independent treatment.
Death detection was confirmed with the vital staining dye neutral red as described by Wendehenne et al.22 Cell cultures (1 mL) were washed with 1 ml of a solution containing 175 mM mannitol, 0.5 mM CaCl2, 0.5 mM K2SO4 and 2 mM Hepes, pH 7.0 and incubated for 5 min in the same solution supplemented with neutral red to a final concentration of 0.01%. Cells with no accumulation of neutral red were considered as dead cells.
H2O2 measurement.
H2O2 released in the culture medium was quantified at different times by measuring the chemiluminescence of luminol reacting with H2O2.29 The infected culture medium (200 µL) was added to 600 µL phosphate buffer (50 mM, pH 7.9) prior to addition of 100 µL luminol 1.1 mM (and 100 µL K3[Fe(CN)6 14 mM]. Chemiluminescence was monitored at 5 min interval with a FB12-Berthold luminometer, with a signal integrating time of 0.2 s.
Camalexin determination.
Camalexin determination was performed as described by Glazebrook and Ausubel.30 For each sample, 20 mL of the infected suspension cells were filtered and heated in 350 µL of 80% methanol for 20 min. The cells were removed and the methanol was evaporated under vacuum. The aqueous residue was extracted with two 50 µL aliquots of chloroform, which were combined and evaporated to dryness. The residue was dissolved in 100 µL chloroform, applied to silica thin-layer chromatography plates and developed in ethyl acetate hexane 9:1 (vol/vol). Camalexin (Rf 0.81) was visualized by its blue fluorescence under a long-wave ultraviolet lamp (365 nm). The silica containing camalexin was scraped off the plate and camalexin was extracted into 1 mL ethanol. The emission at 385 nm after excitation at 315 nm was measured with a Hitachi F2000 fluorimeter and camalexin concentration was calculated by comparison with a standard curve obtained by using purified camalexin kindly provided by Jane Glazebrook (Torrey Mesa Research Institute, San Diego, California 92121).
Statistics.
Mean separation was accomplished using Duncan's Multiple Range Test. Statistical significance was determined at p values <0.05.
Acknowledgements
The authors wish to thank Dr. Glazebrook for the kind donation of purified camalexin used for the standard curve. Thanks also go to the LEM team (EA 3514) for technical facilities. This work was supported by MENESR via the EA 3495.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5545
References
- 1.Riches CR, Parker C. Parasitic plants. London: Chapman & Hall; 1995. pp. 226–255. [Google Scholar]
- 2.Schneeweiss GM. Correlated evolution of life history and host range in the nonphotosynthetic parasitic flowering plants Orobanche and Phelipanche (Orobanchaceae) J Evol Biol. 2007;20:471–478. doi: 10.1111/j.1420-9101.2006.01273.x. [DOI] [PubMed] [Google Scholar]
- 3.Abu Irmaileh BE. Present status of Orobanche control in the near east. In: Wegmann K, Musselman LJ, Joel DM, editors. Current Problems in Orobanche Researches; Proceedings of the 4th International Orobanche Workshop; September 23–26; Albena, Bulgaria. 1998. pp. 425–430. [Google Scholar]
- 4.Musselman LJ, Yoder JI, Westwood JH. Parasitic plants major problem to food crops. Science. 2001;293:1434. doi: 10.1126/science.293.5534.1434a. [DOI] [PubMed] [Google Scholar]
- 5.Benharrat H, Boulet C, Theodet, Thalouran P. Virulence diversity among branched broomrape (O. ramosa L.) populations in France. Agron Sustain Dev. 2005;25:123–128. [Google Scholar]
- 6.Boone L, Lynn DG. Signal transduction in the initiation of germination. In: Press MC, Graves JD, editors. Parasitic flowering plants. London: Chapman & Hall; 1995. pp. 14–38. [Google Scholar]
- 7.Lynn DG, Chang M. Phenolic signals in cohabitation: implications for plant development. Ann Rev Plant Physiol Plant Mol Biol. 1990;41:497–526. [Google Scholar]
- 8.Matvienko M, Torres M, Yoder J. Transcriptional responses in the hemiparasitic plant Triphysaria versicolor to host plant signals. Plant Physiol. 2001;127:272–282. doi: 10.1104/pp.127.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoder JI. Host-plant recognition by parasitic Scrophulariaceae. Curr Opin Plant Biol. 2001;4:359–365. doi: 10.1016/s1369-5266(00)00185-0. [DOI] [PubMed] [Google Scholar]
- 10.Joel DM, Portinoy VH. The Angiospermous root parasite Orobanche L. (Orobanchaceae) induces expression of a pathogenesis related (PR) gene insusceptible tobacco roots. Ann Bot. 1998;81:779–781. [Google Scholar]
- 11.Westwood JH, Yu X, Foy CL, Cramer CL. Expression of a defense-related 3-hydroxy-3-methylglutaryl CoA reductase gene in response to parasitization by Orobanche spp. Mol Plant Microbe Interact. 1998;11:530–536. doi: 10.1094/MPMI.1998.11.6.530. [DOI] [PubMed] [Google Scholar]
- 12.Gowda BS, Riopel JL, Timko MP. NRSA-1: A resistance gene homolog expressed in roots of non-host plants following parasitism by Striga asiatica (witchweed) Plant J. 1999;20:217–230. doi: 10.1046/j.1365-313x.1999.00598.x. [DOI] [PubMed] [Google Scholar]
- 13.Werner M, Uehlein N, Proksch P, Kaldenhoff R. Characterization of two tomato aquaporins and expression during incompatible interaction of tomato with the plant parasite Cuscuta reflexa. Planta. 2001;213:550–555. doi: 10.1007/s004250100533. [DOI] [PubMed] [Google Scholar]
- 14.Castillejo MA, Amiour N, Dumas Gaudot E, Rubiales D, Jorrín JV. A proteomic approach to studying plant response to crenate broomrape (Orobanche crenata) in pea (Pisum sativum) Phytochem. 2004;65:1817–1828. doi: 10.1016/j.phytochem.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 15.Vieira Dos Santos C, Letousey P, Delavault P, Thalouarn P. Defense gene expression analysis of Arabiopsis thaliana parasitized by Orobanche ramosa. Phytopathol. 2003;93:451–458. doi: 10.1094/PHYTO.2003.93.4.451. [DOI] [PubMed] [Google Scholar]
- 16.Vieira Dos Santos C, Delavault P, Letousey P, Thalouarn P. Identification by suppression subractive hybridization and expression analysis of A. thaliana putative defence genes during O. ramosa infection. Physiol Mol Plant Pathol. 2003;62:297–303. [Google Scholar]
- 17.Gibot Leclerc S, Brault M, Sallé G. L'orobanche rameuse, la menace s'aggrave pour le colza, le chanvre et le tabac. Phytoma. 2003;561:9–12. [Google Scholar]
- 18.Atkinson MM, Midland SL, Sims JJ, Keen NT. Syringolide 1 triggers Ca2+ influx, K+ efflux, and extracellular alkalization in soybean cells carrying the disease-resistance gene Rpg4. Plant Physiol. 2004;112:297–302. doi: 10.1104/pp.112.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouizgarne B, El-Maarouf Bouteau H, Frankart C, Reboutier D, Madiona K, Pennarun AM, Monestiez M, Trouverie J, Amiar Z, Briand J, Brault M, Rona JP, Ouhdouch Y, El Hadrami I, Bouteau F. Early physiological responses of Arabidopsis thaliana cells to fusaric acid: toxic and signaling effects. New Phytol. 2006;169:209–218. doi: 10.1111/j.1469-8137.2005.01561.x. [DOI] [PubMed] [Google Scholar]
- 20.Levine A, Pennell RI, Alvarez ME, Palmer R, Lamb C. Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr Biol. 1996;6:427–437. doi: 10.1016/s0960-9822(02)00510-9. [DOI] [PubMed] [Google Scholar]
- 21.Naton B, Hahlbrock K, Schmelzer E. Correlation of rapid cell death with metabolic changes in fungus-infected, cultured parsley cells. Plant Physiol. 1996;112:433–444. doi: 10.1104/pp.112.1.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wendehenne D, Lamotte O, Frachisse JM, Barbier Brygoo H, Pugin A. Nitrate efflux is an essential component of the cryptogein signaling pathway leading to defense responses and hypersensitive cell death in tobacco. Plant Cell. 2002;14:1937–1951. doi: 10.1105/tpc.002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neill S, Desikan R, Hancock J. Hydrogen peroxide signalling. Curr Opin Plant Biol. 2002;5:388–395. doi: 10.1016/s1369-5266(02)00282-0. [DOI] [PubMed] [Google Scholar]
- 24.Johnson AW, Gowda G, Hassanali A, Knox J, Monaco S, Razavi Z, Rosebery G. The preparation of synthetic analogues of strigol. J Chem Soc Perkin Trans. 1981:1734. [Google Scholar]
- 25.Gamborg OL, Miller RA, Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968;50:151. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- 26.El Maarouf H, Barny MA, Rona JP, Bouteau F. Harpin, a hypersensitive response elicitor from Erwinia amylovora, regulates ion channel activities in Arabidopsis thaliana suspension cells. FEBS Lett. 2001;497:82–84. doi: 10.1016/s0014-5793(01)02441-3. [DOI] [PubMed] [Google Scholar]
- 27.Reboutier D, Bianchi M, Brault M, Roux C, Dauphin A, Rona JP, Legue V, Lapeyrie F, Bouteau F. The indolic compound hypaphorine produced by ectomycorrhizal fungus interferes with auxin action and evokes early responses in nonhost Arabidopsis thaliana. Mol Plant Microbe Interact. 2002;15:932–938. doi: 10.1094/MPMI.2002.15.9.932. [DOI] [PubMed] [Google Scholar]
- 28.Levine A, Tenhaken R, Dixon RA, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive responses disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- 29.Jabs T, Tschöpe M, Colling C, Hahlbrock K, Scheel D. Elicitor-stimulated ion fluxes and O2- from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc Natl Acad Sci USA. 1997;94:4800–4805. doi: 10.1073/pnas.94.9.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glazebrook J, Ausubel FM. Isolation of phytoalexin-deficient mutants of A. thaliana and characterization of their interactions with bacterial pathogens. Proc Natl Acad Sci USA. 1994;91:8955–8959. doi: 10.1073/pnas.91.19.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glawischnig E. Camalexin. Phytochemistry. 2007;68:401–406. doi: 10.1016/j.phytochem.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Desikan R, Reynolds A, Hancock JT, Neill SJ. Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defence gene expression in Arabidopsis suspension cultures. Biochem J. 1998;330:115–120. doi: 10.1042/bj3300115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desikan R, Neill SJ, Hancock JT. Hydrogen peroxide-induced gene expression in A. thaliana. Free Radic Biol Med. 2000;28:773–778. doi: 10.1016/s0891-5849(00)00157-x. [DOI] [PubMed] [Google Scholar]
- 34.Desikan R, Hancock JT, Neill SJ, Coffey MJ, Jones OT. Elicitor-induced generation of active oxygen in suspension cultures of A. thaliana. Biochem Soc Trans. 1996;24:199. doi: 10.1042/bst024199s. [DOI] [PubMed] [Google Scholar]
- 35.Clarke A, Desikan R, Hurst RD, Hancock JT, Neill SJ. NO way back: nitric oxide and programmed cell death in Arabidopsis thaliana suspension cultures. Plant J. 2000;24:667–677. doi: 10.1046/j.1365-313x.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- 36.Karpinski S, Reynolds H, Karpinska B, Wingsle G, Mullineaux P. Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science. 1999;284:654–657. doi: 10.1126/science.284.5414.654. [DOI] [PubMed] [Google Scholar]
- 37.Mittler R, Simon L, Lam E. Pathogen-induced programmed cell death in tobacco. J Cell Sci. 1997;110:1333–1344. doi: 10.1242/jcs.110.11.1333. [DOI] [PubMed] [Google Scholar]
- 38.Dörr I, Kollmann R. Strukturelle Grundlagen des Parasitismus bei Orobanche. I. Wachstum der Haustorialzellen im Wirtsgewebe. Protoplasma. 1974;80:245–259. [Google Scholar]
- 39.Attawi FAJ, Weber HC. Zum Parasitismus und zur morphologischen-anatomischen Struktur der Sekundas rhaustorium von Orobanche-Arten (Orobanchaceae) Flora. 1980;169:55–83. [Google Scholar]
- 40.Aber M. Cyto-physiological aspects of Orobanche crenata parasitizing Vicia faba. In: Parker C, Musselman LJ, Polhil RM, Wilson AK, editors. Proceedings of the 3rd International Symposium of Parasitic Weeds; ICARDA; Aleppo. 1984. pp. 200–209. [Google Scholar]
- 41.Govrin EM, Levine A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol. 2000;10:751–757. doi: 10.1016/s0960-9822(00)00560-1. [DOI] [PubMed] [Google Scholar]
- 42.Ben Hod G, Losner D, Joel DM, Mayer AM. Pectin methyl-esterase in calli and germinating seeds of Orobanche aegyptiaca. Phytochem. 1993;32:1399–1402. [Google Scholar]
- 43.Losner Goshe D, Partnoy VH, Mayer AM, Joel DM. Pectolytic activity by the haustorium of the parasitic plant Orobanche L. (Orobanchaceae) in host roots. Annals Bot. 1998;81:319–326. [Google Scholar]
- 44.Singh A, Singh M. Cell wall degrading enzymes in Orobanche aegyptiaca and its host Brassica campestris. Physiol Plant. 1993;89:177–181. [Google Scholar]
- 45.Zuppini A, Navazio L, Sella L, Castiglioni C, Favaron F, Mariani P. An endopolygalacturonase from Sclerotinia sclerotiorum induces calcium-mediated signaling and programmed cell death in soybean cells. Mol Plant Microbe Interact. 2005;180:849–855. doi: 10.1094/MPMI-18-0849. [DOI] [PubMed] [Google Scholar]
- 46.Nurnberger T, Brunner F, Kemmerling B, Piater L. Innate immunity in plants and animals: striking similarities and obvious differences. Immunol Rev. 2004;198:249–266. doi: 10.1111/j.0105-2896.2004.0119.x. [DOI] [PubMed] [Google Scholar]
- 47.Parker JE. Plant recognition of microbial patterns. Trends Plant Sci. 2003;8:245–247. doi: 10.1016/S1360-1385(03)00105-5. [DOI] [PubMed] [Google Scholar]