Abstract
Ascorbate peroxidase (APX) is a reactive oxygen species (ROSs) scavenging enzyme involved in regulation of intracellular ROS levels by reduction of H2O2 to water using ascorbate as an electron donor. In New Phytologist 2007; 175:462–71, we identified a cotton cytosolic APX1 (GhAPX1) that was significantly accumulated during the fast fiber-cell elongation period, through a proteomics approach. Both the transcript levels of GhAPX1 and the total APX activity were highly induced in response to in vitro applied H2O2 or ethylene. Further analysis showed that ethylene promoted H2O2 production 1 day after it was included in the culture medium, suggesting that H2O2 induced cell elongation processes may be placed downstream of the ethylene signal transduction pathway. In this addendum, quantitative real-time RT-PCR showed that only cytosolic APX1, not other cotton APX genes including a second cytosolic APX2, a glyoxysomal and a stromal APXs, was up-regulated during fiber cell elongating. Exogenous H2O2 was found to induce ethylene production if wild-type cotton ovules were cultured for a longer period of time, implying that there was a feedback regulatory mechanism from H2O2 to ethylene biosynthesis in modulating cotton fiber development.
Key words: reactive oxygen species, ascorbate peroxidase, Gossypium hirsutum, ethylene
Reactive oxygen species (ROSs) including superoxide radicals, hydrogen peroxide, and hydroxyl radicals are formed by successive one-electron reductions of molecular oxygen. Interestingly, it has been proposed that a cross-talk between various ROSs might contribute to stabilize plants under different stress conditions.1 ROS produced in plant is mainly hydrogen peroxide (H2O2) that is relatively stable and electron-neutral. H2O2 usually acts as signaling molecules in programmed cell death, in regulation of photosynthesis and perception of environmental stresses as well as in response to pathogen invasions.2 Excess amounts of H2O2 are known to cause oxidative damages to the host cells. ROS was involved in regulation of plant cell expansion since an Arabidopsis mutant deficient in NADPH oxidase activity showed significantly stunted root hair growth.3 ROS may exert its multi-facet effects via a complex network.2,4
Cotton is the most prevalent natural fiber used in textile industry and is one of the mainstays of Chinese as well as global economy. Cotton lint, or commonly known as cotton fiber, are single-celled trichomes evolved from the ovule epidermis and are perhaps the longest single cells in higher plants. Upland cotton (Gossypium hirsutum L.) generally grows up to 30–40 mm in length, about 15 µm in thickness at full maturity and accounts for more than 90% of the production in the world.5–8 Ascorbate peroxidase (APX, EC, 1.11.1.11), one of the most important antioxidant enzymes in higher plants, utilizes ascorbate as electron donors to reduce H2O2 into water. APX, comprising a family of isozymes in different subcellular compartments, has a high affinity towards H2O2. Cytosolic, chloroplastic, mitochondrial and microsomal (glyoxysomal/peroxisomal) APX isoforms have been characterized in Arabidopsis.9 Cytosolic APX1 was found to play an essential role for cross-compartment protection and maintenance of the cellular reactive oxygen network whereas APX2 is activated by variable stresses.3,10,11 Chloroplastic APXs protect the photosynthetic apparatus against oxidation while microsomal APXs are involved in detoxifying H2O2 produced by fatty acid β-oxidation, especially during seed germination and photorespiration.12,13 In addition to peroxisomal APX, peroxisomal membrane-bound monodehydroascorbate reductase 4 was important for scavenging H2O2 molecules that escaped the peroxisomes.14
Although many APX isozymes have been identified from many higher plants, their functions in regulation of plant growth and development remain elusive. We recently identified and characterized the cotton cytosolic APX1 (APX1) gene that played an important role in modulating fiber cell elongating. Here, we obtained and submitted three additional putative full-length cDNAs encoding cotton APXs to GenBank, including a cytosolic APX2 (Genebank accession no. EU244476), a glyoxysomal APX (Genebank accession no. EU244478) and a stromal APX (Genebank accession no. EU244477). We examined their expression profiles using QRT-PCR (Fig. 1) and found that only APX1 exhibited a strong upregulation at 5 day post-anthesis (dpa) in comparison with its transcript level at 0 dpa ovules. Since the cytosol is an important location for cellular communication among different subcelluar compartments, our data, therefore, seems to suggest that cytosolic GhAPX1 may have a regulatory function in controlling the overall H2O2 level inside a plant cell. The current finding agrees with previous report that an Arabidopsis mutant deficient in cytosolic APX1 displayed a stunted growth phenotype,10,15 although a different study found that both the cytosolic and thylakoidal APXs were involved in subcellular communications from different compartments during abiotic stresses.16
Figure 1.
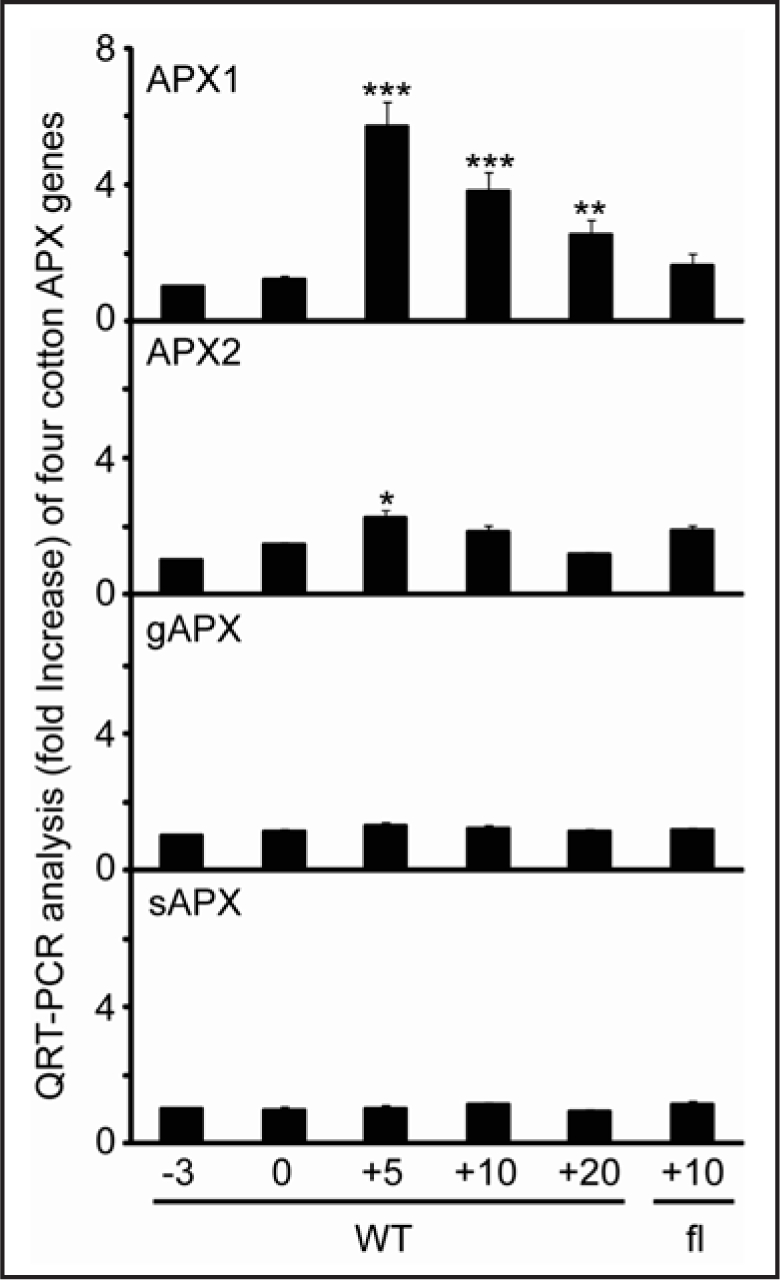
Analysis of transcript levels of various GhAPX genes during different developmental stages. QRT-PCR was performed using RNA samples prepared from triplicate cotton materials harvested from indicated growth stages. The cotton ubiquitin gene, UBQ7 (Genebank accession no. AY189972) was included as a loading control. APX1, cytosolic APX1 (Genebank accession no. EF432582); APX2, cytosolic APX2 (Genebank accession no. EU244476); gAPX, glyoxysomal APX (Genebank accession no. EU244478); sAPX, stromal APX (Genebank accession no. EU244477).
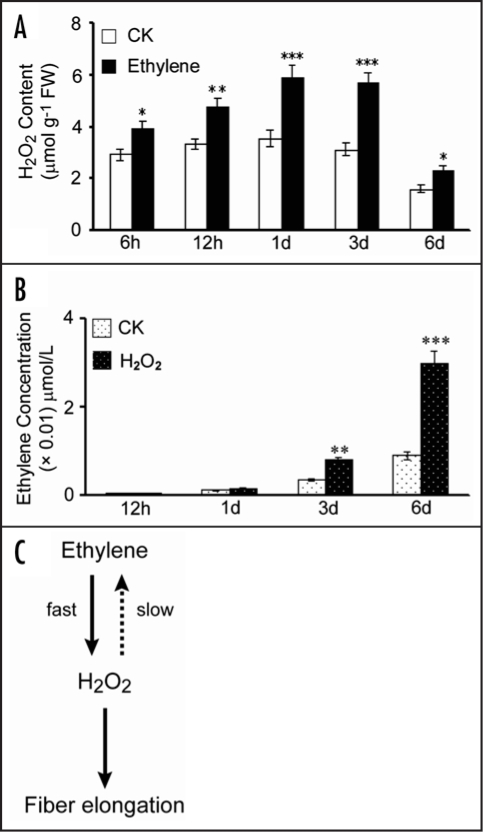
Ethylene was shown to significantly promote fiber growth.17 In vitro applied ethylene enhanced significant H2O2 production as early as 6 h until 1 d when reached a peak value (Fig. 2A), whereas, exogenous H2O2 was as well able to stimulate a significant ethylene production but after 1 d (Fig. 2B). Ethylene or hydrogen peroxide was found to regulate the expression of a soybean ascorbate peroxidase gene while abscisic acid was active in inducing H2O2 production.18,19 H2O2-activated Ca2+ channels were important for stomatal movement.19 Similar studies revealed that ethylene-induced stomatal closure in Arabidopsis depended on H2O2 production. Both ethylene and H2O2 signaling in guard cells were mediated by ethylene receptors.20 Auxin was found to stimulate the biosynthesis of H2O2 during root gravity responses.21 H2O2 generated through NADPH oxidase and superoxide dismutase was shown necessarily for regurgitant-induced increase of ethylene production.22 Taken together, we propose the existence of a regulatory mechanism between H2O2 and ethylene to modulate cotton fiber and may be other related types of cell elongation (Fig. 2C). How plant hormones and ROS interact to regulate the expression of various APX isoenzymes during plant growth and development requires further investigation.
Figure 2.
Ethylene and H2O2 interact to regulated cotton fiber elongation. (A) Exogenous ethylene induced H2O2 production starting from an early stage. (B) Exogenous H2O2 slowly stimulated ethylene production. (C) A proposed model for a regulatory mechanism between H2O2 and ethylene signaling in controlling fiber elongation. Wide-type ovules were harvested at 1 dpa and in vitro cultured in the medium (CK), in presence of 50 µM H2O2 or 0.1 µM ethylene for the time (h or d) indicated. H2O2 content was measured using titanium oxidation method (23). gFW, gram fresh weight. Ethylene production from cultured wild-type ovules was determined according to the protocol described (ref. 17). Statistical significances were determined using one-way ANOVA software combined with Tukey's test. *p < 0.05; **p < 0.01; ***p < 0.001.
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (Grant No. 30470171) to Dr. YM Qin and a grant from China National Basic Research Program (Grant 2004CB117302) to Dr. Y.-X. Zhu.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/5208
References
- 1.Laloi C, Stachowiak M, Pers-Kamczyc E, Warzych E, Murgia I, Apel K. Cross-talk between singlet oxygen- and hydrogen peroxide-dependent signaling of stress responses in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2007;104:672–677. doi: 10.1073/pnas.0609063103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, Davies JM, Dolan L. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 4.Carol RJ, Dolan L. The role of reactive oxygen species in cell growth: Lessons from root hairs. J Exp Bot. 2006;57:1829–1834. doi: 10.1093/jxb/erj201. [DOI] [PubMed] [Google Scholar]
- 5.Basra AS, Malik CP. Development of the cotton fiber. Int Rev Cytol. 1984;89:65–113. [Google Scholar]
- 6.Tiwari SC, Wilkins TA. Cotton (Gossypium hirsutum) seed trichomes expand via diffuse growing mechanism. Can J Bot. 1995;73:746–757. [Google Scholar]
- 7.Ji SJ, Lu YC, Li J, Wei G, Liang X, Zhu YX. A β-tubulin-like cDNA expressed specifically in elongating cotton fibers induces longitudinal growth of fission yeast. Biochem Biophys Res Comm. 2002;296:1245–1250. doi: 10.1016/s0006-291x(02)02069-7. [DOI] [PubMed] [Google Scholar]
- 8.Ji SJ, Lu YC, Feng JX, Wei G, Li J, Shi YH, Fu Q, Liu D, Luo JC, Zhu YX. Isolation and analyses of genes preferentially expressed during early cotton fiber development by subtractive PCR and cDNA array. Nucl Acids Res. 2003;31:2534–2543. doi: 10.1093/nar/gkg358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K. Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot. 2002;53:1305–1319. [PubMed] [Google Scholar]
- 10.Davletova S, Rizhsky L, Liang H, Zhong S, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell. 2005;17:268–281. doi: 10.1105/tpc.104.026971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karpinski S, Escorbar C, Karpinska B, Creissen G, Mullineaux PM. Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell. 1997;9:627–640. doi: 10.1105/tpc.9.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danna CH, Bartoli CG, Sacco F, Ingala LR, Santa-María GE, Guiamet JJ, Ugalde RA. Thylakoid-bound ascorbate peroxidase mutant exhibits impaired electron transport and photosynthetic activity. Plant J. 2003;132:2116–2125. doi: 10.1104/pp.103.021717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corpas FJ, Barroso JB, del Rio LA. Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends in Plant Sci. 2001;6:145–150. doi: 10.1016/s1360-1385(01)01898-2. [DOI] [PubMed] [Google Scholar]
- 14.Eastmond PJ. Monodehydroascorbate reductase4 is required for seed storage oil hydrolysis and postgerminative growth in Arabidopsis. Plant Cell. 2007;19:1376–1387. doi: 10.1105/tpc.106.043992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pnueli L, Liang H, Rozenberg M, Mittler R. Growth suppresion, altered stomatal responses, and augumented induction of heat shock proteins in cytosolic ascorbate peroxidase (Apx1)-deficient Arabidopsis plant. Plant J. 2003;34:187–203. doi: 10.1046/j.1365-313x.2003.01715.x. [DOI] [PubMed] [Google Scholar]
- 16.Miller G, Suzuki N, Rizhsky L, Hegie A, Koussevitzky S, Mittler R. Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant Physiol. 2007;144:1777–1785. doi: 10.1104/pp.107.101436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi YH, Zhu SW, Mao XZ, Feng JX, Qin YM, Zhang L, Cheng J, Wei LP, Wang ZY, Zhu YX. Transcriptome profiling, molecular biological, and physiological studies reveal a major role for ethylene in cotton fiber cell elongation. Plant Cell. 2006;18:651–664. doi: 10.1105/tpc.105.040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SC, Kang BG, Oh SE. Induction of ascorbate peroxidase by ethylene and hydrogen peroxide during growth of cultured soybean cells. Mol Cell. 1999;9:166–171. [PubMed] [Google Scholar]
- 19.Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- 20.Desikan R, Last K, Harrett-Williams R, Tagliavia C, Harter K, Hooley R, Hancock JT, Neill SJ. Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. Plant J. 2006;47:907–916. doi: 10.1111/j.1365-313X.2006.02842.x. [DOI] [PubMed] [Google Scholar]
- 21.Joo JH, Bae YS, Lee JS. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol. 2001;126:1055–1060. doi: 10.1104/pp.126.3.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinite I, Gailite A, Ievinsh G. Reactive oxygen and ethylene are involved in the regulation of regurgitant-induced responses in bean plants. J Plant Physiol. 2004;161:191–196. doi: 10.1078/0176-1617-01098. [DOI] [PubMed] [Google Scholar]
- 23.Brennan T, Frenkel C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 1977;59:411–416. doi: 10.1104/pp.59.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]