Abstract
Rice seedlings (Oryza sativa L.) were subjected to low temperature pretreatment (LT-PT; 10°C) for various length of time followed by a 48-h chilling temperature stress (2°C). Chilling tolerance of rice roots was improved with increasing duration of LT-PT, but HT-PT longer than 12 h gave no additional improvement. LT-PT did not change in fatty acid composition in rice roots under the present experimental condition. Alcohol dehydrogenase (ADH) activity and ethanol concentration in the roots were increased with increasing duration of LT-PT up to 12 h, which indicates that LT-PT increased ethanol fermentation in the roots. 4-Methylpyrazole, a potent inhibitor of ADH, reduced the ethanol concentration and the chilling tolerance in the roots. This reduction of the chilling tolerance recovered with exogenously applied ethanol. Ethanol also induced 21- and 33-kD protein synthesis in the roots and these proteins may contribute the improvement of the tolerance. The present research suggests that LT-PT may increase chilling tolerance in rice roots owing to ethanol production, and ethanol may trigger a signal transduction cascade, which might lead to a decrease in membrane damage and injury.
Key words: acclimation, alcohol dehydrogenase, chilling tolerance, ethanol, heat shock protein, low temperature, Oryza sativa
Alcohol dehydrogenase (ADH; EC.1.1.1.1) gene and protein were induced by low temperature in Arabidopsis, maize and rice seedlings.1,2,3 ADH is an enzyme involved in ethanolic fermentation and essential for plants to survive under anaerobic conditions.4,5 However, it is unlikely that the induction of ADH by low temperture is due to a switch from aerobic respiration to anaerobic respiration as reported with anaerobic conditions.2,6 Therefore, it is not clear that biological meanings of the induction of ADH in low temperature conditions.
Rice seedlings (Oryza sativa L. cv. Nipponbare) were subjected to low temperature pretreatment (LT-PT; 10°C) for various length of time (1, 2, 4, 6, 12, 18, 24 h) followed by a 48-h chilling temperature stress (2°C). Chilling tolerance of rice roots was improved with increasing duration of LT-PT, but HT-PT longer than 12 h gave no additional improvement. LT-PT did not change in any fatty acid compositions in rice roots under the present experimental condition. Several plant species, such as oat, rye and spinach increased freezing tolerance due to the increasing unsaturation of fatty acids in plasma membranes, but this cold acclimation process required exposure of these plants to subzero temperature for 2–3 weeks.7,8
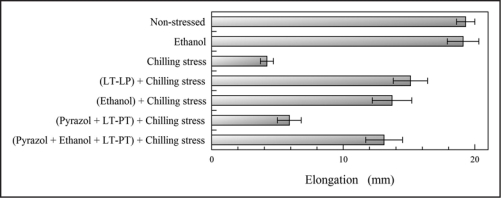
LT-PT increased ADH activity and ethanol concentration in rice roots, and the activity and the concentration were increased with increasing duration of LT-PT up to 12 h. Thus, LT-PT induced ethanolic fermentation system and stimulated ethanol production in the roots. 4-Methylpyrazole, which is a potent inhibitor of ADH and prevents ethanol production,9 reduced rice root growth to 40% of LP-PT root growth (Fig. 1), and the ethanol accumulation in the roots. This growth inhibition by 4-methylpyrazole recovered with exogenously applied ethanol. These results suggest that ethanol produced by LT-PT may contribute the chilling tolerance in the roots of the rice seedlings. In addition, an ADH deficient mutant of maize seedlings, which can not produce ethanol, was more sensitive to chilling temperature than their wild types.6
Figure 1.
Effects of ethanol and 4-methylpyrazole on root growth of rice seedlings. Three-day-old rice seedlings were treated 12-h LT-PT (10°C) with or without 100 mM ethanol and/or 5 mM 4-methylpyrazole at 25°C for 24 h, and then subjected to chilling stress treatment (2°C, 48 h). Elongation of rice roots was determined over 48 h at 25°C after chilling stress treatment. Non-stressed seedlings and ethanol-treated seedlings were grown at 25°C. Chilling stressed seedlings were grown at 25°C for 24 h, and then subjected to chilling stress treatment. Means ± SE from five independent experiments with 20 plants for each determination are shown.
When the seedlings were subjected to chilling temperature stress after ethanol treatment without LT-PT, the growth inhibition of rice roots by chilling temperature recovered from 22% to 71% of that of nonstressed roots (Fig. 1), which suggests that exogenously applied ethanol may improve chilling tolerance in the roots. It is also found that the ethanol treatment did not change in fatty acid composition in the roots at the temperature of this treatment (25°C).
Chilling temperature induced lipid degradation in plant cells of cold-sensitive plants, such as cucumber, rice and soybean, as measured by an increase in malondialdehyde, which is a decomposition product of phospholipid peroxidation.10 Lipid peroxidation occurs when polyunsatured fatty acids are released from phospholipids by phospholipases and became substrates for lipoxygenases. Changes in the structural composition of the plasma membranes by lipid peroxidation cause the phase transition of the membrane from liquid to gel and the inactivation of membrane bound enzymes such as plasma membrane ATPase. Thus, the phase transition of the membranes was thought to be one of the primary causes of chilling injury.11–13
The addition of C1 to C6 alcohols including ethanol to model membranes increased fluidity of the membranes and lowered the phase transition temperature of the membranes.14,15,16 Therefore, ethanol produced by LT-PT may prevent the phase transition of the membrane from liquid to gel, and lower the phase transition temperature of the membranes, which may contribute the acclimation to the chilling tolerance. In addition, ethanol induced an increase in ATPase activity in plasma membranes,6 and prevented chilling-induced ion leakage from plant tissues.17
Ethanol is also known to stimulate the synthesis of heat shock protein (HSP) in yeast, bacteria and some other plants.18,19 We thus determined the effect of ethanol on protein synthesis in rice roots by SDS-gel electrophoresis, and found that 21- and 33-kD protein synthesis were induced by ethanol. These proteins were also induced by heat shock treatment (45°C, 20 min). HSP was shown to be associated with the development of low temperature tolerance in spinach.20,21 Thus, 21- and 33-kD proteins induced by ethanol may contribute the improvement of the chilling tolerance.
The present research suggests that LT-PT-induced chilling tolerance may be owing to ethanol accumulation in rice roots. Accumulated ethanol may increase the fluidity of plasma membranes and lower the phase transition temperature of the membranes, and may also induce protein synthesis. This hypothesis is supported by exogenously applied ethanol which increased the chilling tolerance. Thus, ethanol might trigger a signal transduction cascade, which would lead to a decrease in membrane damage and injury. Further work needs to be done to test this possibility.
Abbreviations
- ADH
alcohol dehydrogenase
- LP-PT
low temperature pretreatment
- HSP
heat shock protein
Addendum to: Kato-Noguchi H. Low temperature acclimation to chilling tolerance in rice roots. Plant Growth Regul. 2007;51:171–175.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/5542
References
- 1.Jarillo JA, Leyva A, Salinas J, Martínez-Zapater JM. Low temperature induces the accumulation of alcohol dehydrogenase mRNA in Arabidopsis thaliana, a chilling-tolerant plant. Plant Physiol. 1993;101:833–837. doi: 10.1104/pp.101.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dolferus R, Ellis M, de Bruxelles G, Trevaskis B, Hoeren F, Dennis ES, Peacock WJ. Strategies of gene action in Arabidopsis during hypoxia. Ann Bot. 1997;79(Suppl):21–31. [Google Scholar]
- 3.Minhas D, Grover A. Transcript levels of genes encoding various glycolytic and fermentation enzymes change in response to abiotic stresses. Plant Sci. 1999;146:41–51. [Google Scholar]
- 4.Drew MC. Oxygen deficiency and root metabolism. Injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:223–250. doi: 10.1146/annurev.arplant.48.1.223. [DOI] [PubMed] [Google Scholar]
- 5.Tadege M, Dupuis I, Kuhlemeier C. Ethanolic fermentation, new functions for an old pathway. Trends Plant Sci. 1999;4:320–325. doi: 10.1016/s1360-1385(99)01450-8. [DOI] [PubMed] [Google Scholar]
- 6.Peters JS, Frenkel C. Relationship between alcohol dehydrogenase activity and low-temperature in two maize genotypes, Silverado F1 and Adh1- Adh2- doubly null. Plant Physiol Biochem. 2004;42:841–846. doi: 10.1016/j.plaphy.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Uemura M, Steponkus PL. A contrast of the plasma membrane lipid composition of oat and rye leaves in relation to freezing tolerance. Plant Physiol. 1994;104:479–496. doi: 10.1104/pp.104.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomashow MF. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:571–579. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- 9.Perata P, Alpi A. Ethanol-induced injuries to carrot cells. The role of acetaldehyde. Plant Physiol. 1991;95:748–752. doi: 10.1104/pp.95.3.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parkin KL, Kuo SJ. Chilling-induced lipid degradation in cucumber (Cucumis sativus L., cv. Hybrid C) fruit. Plant Physiol. 1989;90:1049–1056. doi: 10.1104/pp.90.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyons JM. Chilling injury in plant. Annu Rev Plant Physiol. 1973;24:445–466. [Google Scholar]
- 12.Yoshida S. Low temperature-induced cytoplasmic acidosis in cultured mung bean (Vigna radiate (L.) Wilczek) cells. Plant Physiol. 1994;104:1131–1138. doi: 10.1104/pp.104.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng AL, Tang X, Wang X. Changes of microsomal membrane properties in spring wheat leaves (Triticum aestivum L.) exposed to enhanced and ultraviolet-B radiation. J Photochem Photobiol. 2000;57:60–65. doi: 10.1016/s1011-1344(00)00077-4. [DOI] [PubMed] [Google Scholar]
- 14.Ingram LO, Buttke TM. Effects of alchol on micro-oganism. Adv Microb Physiol. 1984;25:253–300. doi: 10.1016/s0065-2911(08)60294-5. [DOI] [PubMed] [Google Scholar]
- 15.Chou JS, Ma SM, Kamaya H, Ueda I. Anesthesia cutoff phenomenon: Interfacial hydrogen bonding. Science. 1990;248:583–585. doi: 10.1126/science.2159183. [DOI] [PubMed] [Google Scholar]
- 16.Frenkel C, Erez A. Induction of chilling tolerance in cucumber (Cucumis sativus) seedlings by endogenous and applied ethanol. Physiol Plant. 1996;96:593–600. [Google Scholar]
- 17.Saltveit ME. Exposure to alcohol vapours reduces chilling-induced injury of excised cucumber cotyledons, but not of seedlings or excised hypocotyls segments. J Exp Bot. 1994;45:813–821. [Google Scholar]
- 18.Sanchez Y, Taullen J, Borkovich RA, Lindquist S. Hsp 104 is required for tolerance to many forms of stress. EMBO J. 1992;11:2357–2364. doi: 10.1002/j.1460-2075.1992.tb05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saltveit ME, Peiser G, Rab A. Effect of acetaldehyde, arsenite, ethanol, and heat shock on protein synthesis and chilling sensitivity of cucumber radicles. Physiol Plant. 2004;120:556–562. doi: 10.1111/j.1399-3054.2004.0280.x. [DOI] [PubMed] [Google Scholar]
- 20.Anderson JV, Li QB, Haskel DW, Guy CL. Structural organization of the spinach endoplasmic reticulum-luminal 70-kilodalton heat shock cognate gene and expression 70-kilodaltone heat shock genes during cold acclimation. Plant Physiol. 1994:1359–1370. doi: 10.1104/pp.104.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guy CL. Cold acclimation and freezing stress tolerance: Role of protein metabolism. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:187–223. [Google Scholar]