Abstract
Copper amine oxidases (CuAO) and flavin-containing amine oxidases (PAO) are hydrogen peroxide (H2O2)-producing enzymes responsible for the oxidative de-amination of polyamines. Currently, a key role has been ascribed to apoplastic amine oxidases in plants, i.e., to behave as H2O2-delivering systems in the cell wall during cell growth and differentiation as well as in the context of host-pathogen interactions. Indeed, H2O2 is the co-substrate for the peroxidase-driven reactions during cell-wall maturation and a key signalling molecule in defence mechanisms. We recently demonstrated the involvement of an apoplastic PAO in the wound-healing process of the Zea mays mesocotyl. Experimental evidence indicated a similar role for an apoplastic PAO in Nicotiana tabacum. In this addendum we suggest that a CuAO activity is also involved in this healing event.
Key words: polyamine oxidase, copper amine oxidase, hydrogen peroxide, wound healing
Amine oxidases (AO) are an heterogeneous group of enzymes including CuAO and PAO, which oxidize polyamines at the primary and secondary amino group, respectively. Identity of reaction products varies depending on substrate type and mode of oxidation. However, H2O2 is a shared product in all the AO-mediated oxidations.1
In plants, several biological functions ascribed to AO have been linked to the production of H2O2. Indeed, it has been suggested that important developmental and defence responses depend on the apoplastic production of H2O2 carried out by cell wall-localized CuAO and PAO through polyamine oxidation.1 Polyamine-derived H2O2 is involved in cell wall differentiation by driving peroxidase-mediated oxidative cross-linking of cell wall components2,3 or behaves as second messenger in signalling programmed cell death (PCD) such as, for instance, developmental PCD during vascular differentiation in Arabidopsis (Arabidopsis thaliana)4 or hypersensitive response (HR) cell death in tobacco (Nicotiana tabacum) plants infected with tobacco mosaic virus.5 However, CuAO mediated-H2O2 production has also been linked to cell expansion in fast-growing tissues of soybean seedlings, inasmuch as the simultaneous apoplastic production of superoxide anion (O2-.) can lead to peroxidase-mediated H2O2 reduction to form the wall-loosening agent hydroxyl radical (·OH).6
CuAO and PAO, due to their abundance in the apoplast of Leguminosae and Gramineae respectively, have been long considered distinctive enzymes of these two plant families,7 leaving open the question concerning polyamine catabolism in other plant families and/or cellular compartments. More recently, genetic, biochemical and molecular approaches supported by data-base analysis revealed the presence of a number of putative CuAO and PAO in several plant species,1 belonging to different plant families. Moreover, prediction of subcellular localization based on the analysis of primary structures suggested the occurrence of AO also in intracellular compartments. In Arabidopsis five cDNA encoding for putative PAO8 and ten cDNA encoding for putative CuAO (Cona unpublished) can be retrieved by database search, only a few of which showing apoplastic localization. In barley (Hordeum vulgare) two paralogous PAO genes code for two protein isoforms, namely the likely apoplastic HvPAO19 and the vacuolar HvPAO2.10 Moreover, a CuAO activity has been involved in the supply of H2O2 to peroxidase-catalysed reactions during grain filling in the same plant.11 In maize (Zea mays), three PAO gene copies encoding for identical apoplastic proteins have been characterized.12 This growing information about physiological, biochemical and molecular features of AO in the plant kingdom, made evident the complexity of polyamine catabolism. The apparent redundancy of AO in plants could reflect the need for compartment-specific H2O2 synthesis in different phases of development and differentiation as well as in the course of defence mechanisms.
By means of both pharmacological and genetic approaches, we have recently demonstrated that PAO, similarly to what previously demonstrated for CuAO in chickpea (Cicer arietinum) plants,2 plays a role in wound-healing by delivering the H2O2 required for suberin polyphenolic domain and lignin synthesis catalyzed by peroxidase.13 The involvement of Zea mays PAO (ZmPAO)-mediated H2O2 production in wound-healing events was analyzed both in wounded maize mesocotyl by exploiting the in vivo use of N-prenylagmatine (G3), a specific and selective ZmPAO inhibitor, as well as in transgenic tobacco plants constitutively expressing high level of ZmPAO in the cell wall. These experiments indicated that G3 strongly inhibited lignin and suberin polyphenolic domain deposition along the wound periderm in maize mesocotyl and that ZmPAO over-expression in tobacco cell wall greatly accelerates this phenomenon in wounded tobacco stem.13 As expected, treatment of wounded tobacco plants with the ZmPAO substrate spermidine, enhanced lignosuberized deposition in transgenic-tobacco plants.13 However, spermidine had a similar effect also in wild type-tobacco plants,13 suggesting that an endogenous amine oxidase could be involved in wound-healing processes in tobacco plants.
Being spermidine one of the preferential ZmPAO substrate, the identity of the endogeneous AO conceivably appeared to be a PAO, likely the cell-wall localized Nitcotiana tabacum PAO (NtPAO) previously demonstrated to be involved in defence response against pathogens.5
However, we unavoidable must consider that spermidine can be oxidized also by CuAO, although with a lower catalytic constant, and that a cell-wall localized CuAO activity has been reported to be involved in defence response against pathogens in tobacco plants as well.14 Consequently, we explored the possibility that CuAO participates to wound-healing in tobacco, by treating wounded tobacco plants with putrescine (Put), an exclusive CuAO substrate, and two CuAO inhibitors, aminoguanidine (Fig.1) and 2-bromoethylamine (not shown). 8-Week-old tobacco (Nicotiana tabacum cv Petit Havana SR1) plants were blade-wounded on the second internode (numbered from shoot apex). After injury, some plants were treated with 1 x 10-3 M Put in 0.01 M sodium phosphate buffer, pH 6.5, by brushing 50 µL of the polyamine solution along the wound every 24 h after wounding. Others plants were treated with 1 x 10-3 M aminoguanidine or 5 x 10-3 M 2-bromoethylamine in the presence or absence of Put. After 72 h from injury, cross sections of wounded internodes from treated and control plants, were examined for UV-light induced yellow autofluorescence, as indicative of ligno-suberization level along wound periderm cell walls, accordingly to Sherf et al.15 The presence of a strong yellow autofluorescence in the innermost part of the wound periderm of Put-treated plants (Fig. 1B), not yet detectable in untreated plants (Fig. 1A), indicates that this polyamine accelerates deposition of lignin and suberin polyphenolic domain along the wound, presumably through CuAO-mediated H2O2 delivering in the cell wall. The absence of yellow autofluorescence in cross section of plants simultaneously treated with Put and aminoguanidine (Fig. 1C) or 2-bromoethylamine (not shown), revealed that CuAO inhibition blocks Put-induced ligno-suberization along wound periderm, suggesting the involvement of an apoplastic CuAO activity in the cicatrisation process.
Figure 1.
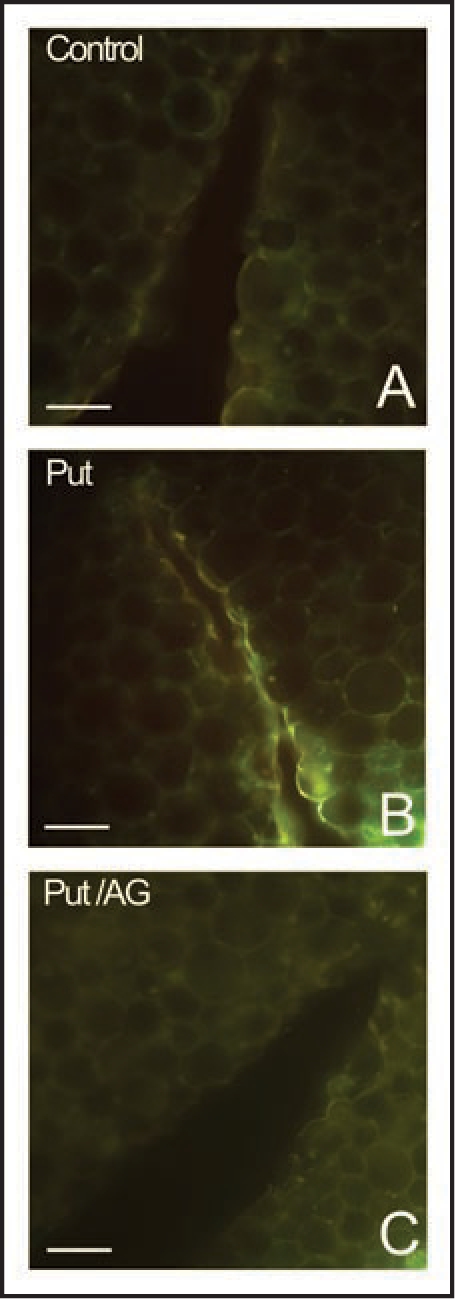
UV-induced autofluorescence microscopic analysis in wound-healing tobacco plants. Histochemical analysis was performed in hand-cut cross sections (∼100 µm thick) obtained from the wounded zone of the second internode (numbering from the shoot apex) at 72 h after wounding. Sections were directly mounted on slides and observed for autofluorescence under UV light. (A) Put-untreated plants (Control); (B) Put-treated plants; (C) plants simultaneously treated with Put and aminoguanidine (AG). Bar = 100 µm.
Presently, a number of evidence suggest the occurrence of both CuAO and PAO activity in the same cellular compartment of tobacco plants. Moreover, our experiments seem to suggest that these enzymes could be active at the same time in the same tissues. In our opinion, the likely simultaneous presence of CuAO and PAO activity in tobacco cell wall indicate either the need of a composite control on polyamine catabolism-dependent H2O2 production, or, analogously to what previously suggested in tobacco vascular tissues undergoing lignification,16 the occurrence of a cooperation of these two enzymes in the AO-mediated oxidative burst during plant defence responses following mechanical damage or pathogen attack.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5372
References
- 1.Cona A, Rea G, Angelini R, Federico R, Tavladoraki P. Functions of amine oxidases in plant development and defense. Trends Plant Sci. 2006;11:80–88. doi: 10.1016/j.tplants.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Rea G, Metoui O, Infantino A, Federico R, Angelini R. Copper amine oxidase expression in defense responses to wounding and Ascochyta rabiei invasion. Plant Physiol. 2002;128:865–875. doi: 10.1104/pp.010646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cona A, Cenci F, Cervelli M, Federico R, Mariottini P, Moreno S, Angelini R. Polyamine oxidase, a hydrogen peroxide-producing enzyme, is upregulated by light and downregulated by auxin in the outer tissues of the maize mesocotyl. Plant Physiol. 2003;131:803–813. doi: 10.1104/pp.011379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Møller SG, McPherson MJ. Developmental expression and biochemical analysis of the Arabidopsis atao1 gene encoding an H2O2-generating diamine oxidase. Plant J. 1998;13:781–791. doi: 10.1046/j.1365-313x.1998.00080.x. [DOI] [PubMed] [Google Scholar]
- 5.Yoda H, Yamaguchi Y, Sano H. Induction of hypersensitive cell death by hydrogen peroxide produced through polyamine degradation in tobacco plants. Plant Physiol. 2003;132:1973–1981. doi: 10.1104/pp.103.024737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delis C, Dimou M, Flemetakis E, Aivalakis G, Katinakis P. A root- and hypocotyls-specific gene coding for copper-containing amine oxidase is related to cell expansion in soybean seedlings. J Exp Bot. 2006;1:101–111. doi: 10.1093/jxb/erj009. [DOI] [PubMed] [Google Scholar]
- 7.Slocum RD, Furey MJ. Electron-microscopic cytochemical localization of diamine and polyamine oxidases in pea and maize tissues. Planta. 1991;183:443–450. doi: 10.1007/BF00197744. [DOI] [PubMed] [Google Scholar]
- 8.Tavladoraki P, Rossi MN, Saccuti G, Perez Amador MA, Polticelli F, Angelini R, Federico R. Heterologous expression and biochemical characterization of a polyamine oxidase from Arabidopsis involved in polyamine back conversion. Plant Physiol. 2006;141:1519–1532. doi: 10.1104/pp.106.080911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cervelli M, Bianchi M, Cona A, Crosatti C, Stanca M, Angelini R, Federico R, Mariottini P. Barley polyamine oxidase isoforms 1 and 2, a peculiar case of gene duplication. FEBS J. 2006;273:3990–4002. doi: 10.1111/j.1742-4658.2006.05402.x. [DOI] [PubMed] [Google Scholar]
- 10.Cervelli M, Di Caro O, Di Penta A, Angelini R, Federico R, Vitale A, Mariottini P. A novel C-terminal sequence from barley polyamine oxidase is a vacuolar sorting signal. Plant J. 2004;40:410–418. doi: 10.1111/j.1365-313X.2004.02221.x. [DOI] [PubMed] [Google Scholar]
- 11.Asthir B, Duffus CM, Smith RC, Spoor W. Diamine oxidase is involved in H2O2 production in the chalazal cells during barley grain filling. J Exp Bot. 2002;53:677–682. doi: 10.1093/jexbot/53.369.677. [DOI] [PubMed] [Google Scholar]
- 12.Cervelli M, Tavladoraki P, Di Agostino S, Angelini R, Federico R, Mariottini P. Isolation and characterization of three polyamine oxidase genes from Zea mays. Plant Physiol Biochem. 2000;38:667–677. [Google Scholar]
- 13.Angelini R, Tisi A, Rea G, Chen MM, Botta M, Federico R, Cona A. Involvement of polyamine oxidase in wound healing. Plant Physiol. 2008;146:162–177. doi: 10.1104/pp.107.108902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marini F, Betti L, Scaramagli S, Biondi S, Torrigiani P. Polyamine metabolism is upregulated in response to tobacco mosaic virus in hypersensitive, but not in susceptible tobacco. New Phytol. 2001;149:301–309. doi: 10.1046/j.1469-8137.2001.00017.x. [DOI] [PubMed] [Google Scholar]
- 15.Sherf BA, Bajar AM, Kolattukudy PE. Abolition of an inducible highly anionic peroxidase activity in transgenic tomato. Plant Physiol. 1993;101:201–208. doi: 10.1104/pp.101.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paschalidis KA, Roubelakis Angelakis KA. Sites and regulation of polyamine catabolism in the tobacco plant. Correlations with cell division/expansion, cell cycle progression and vascular development. Plant Physiol. 2005;138:2174–2184. doi: 10.1104/pp.105.063941. [DOI] [PMC free article] [PubMed] [Google Scholar]


