Abstract
Under abiotic stress conditions, rapid increases in reactive oxygen species (ROS) levels occurs within plant cells. Although their role as a major signalling agent in plants is now acknowledged, elevated ROS levels can result in an impairment of membrane integrity. Similar to our previous findings on imposition of salt stress, application of the hydroxyl radical (OH•) to Arabidopsis roots results in a massive efflux of K+ from epidermal cells. This is likely to cause significant damage to cell metabolism. Since K+ loss also occurs after salt application and salt stress leads to increased cellular ROS levels, we suggest that at least some of the detrimental effects of salinity is due to damage by its resulting ROS on K+ homeostasis. We also observed a comparative reduction in K+ efflux by compatible solutes after both oxidative and salt stress. Thus, we propose that under saline conditions, compatible solutes mitigate the oxidative stress damage to membrane transporters. Whether this amelioration is due to free-radical scavenging or by direct protection of transporter systems, warrants further investigation.
Key words: compatible solutes, hydroxyl radical, potassium efflux, reactive oxygen species, salt stress
Reactive oxygen species (ROS) are continuously produced as by-products of various metabolic pathways.1 Under unstressed steady-state conditions, cellular ROS levels are kept in check by the sophisticated antioxidant defence system.2 However, under adverse environmental conditions, the balance between ROS production and its subsequent scavenging may be perturbed, leading to a rapid increase in ROS levels.3 Although significant progress has been made in defining ROS as a major signalling agent in plants,3 ROS can react with a large variety of biomolecules, causing lipid peroxidation and impairing membrane integrity.4,5 One such abiotic stress is salt stress,6 with ROS generation occurring within minutes of salt application.7 Alleviation of oxidative damage may be, therefore, an important strategy of plant salt tolerance.8
One of the earliest measurable responses to salt stress is a massive K+ efflux from plant roots.9,10 Such K+ efflux is initiated within seconds of acute salt stress and may last for several hours11,12 reducing the intracellular K+ pool13,14 and significantly impairing cell metabolism. Consistent with the key role of K+ homeostasis in salt tolerance mechanisms15 a reduction of K+ efflux correlates with increased salt tolerance.11,12
We have previously reported that hydroxyl radical (OH•) application to Arabidopsis roots also results in a rapid efflux of K+ from the epidermis.16 In this report, we find a similar K+ efflux response.17 As is the case for salt stress,9 we found that membrane depolarisation could be responsible for a substantial part of this efflux. However, an observed discrepancy between the membrane depolarisation and the pattern of K+ efflux indicates that voltage-dependence is not the only factor influencing K+ loss from the root cells after oxidative stress. Demidchik et al.16 demonstrated that stress-induced K+ efflux could be mediated by activation of K+ outward rectifying channels directly by OH•. This direct effect on K+ transporters could also account for our observed delay before the peak efflux of K+ is measured, indicating that a certain amount of time is required before maximal direct damage by OH• to transporters occurs. Because both K+ channel blockers and non-selective cation channel blockers reduce this efflux, it indicates non-specificity in OH• attack. Furthermore, combinations of these channel blockers were effective in reducing K+ efflux implying that, at least in the short term, the damaging effects of OH• is due to compromising the transporter systems as opposed to lipid peroxidation. Certainly, K+ channels harbour reactive groups, thus are expected to be sensitive to ROS.18
We have previously shown that the exogenous application of low concentrations of a variety of compatible solutes reduces the salt-induced K+ efflux.19,20 Plants, when confronted with a saline environment, respond with a significant elevation in their compatible solute levels. This ameliorates the detrimental effects of salinity.21 However, their original proposed role in cellular osmoregulation is under question: their concentration in transgenic plants overexpressing osmolyte biosynthetic genes is not significant for osmotic adjustment, despite showing improved salt tolerance.8 Furthermore, one hallmark of the detoxification effect is its lack of specificity, that is, transgenic plants have increased tolerance not only to high salt, but also to drought, cold and heat shock,22,23 stresses that also result in ROS production.3 Certainly, ecotopic expression studies suggest that compatible solutes increase stress tolerance by protection of membranes and proteins against ROS.6
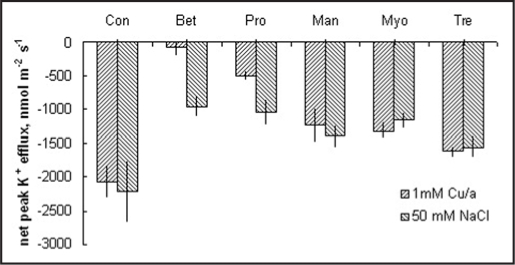
We show that in this work that exogenous application of low concentrations of a range of compatible solutes significantly reduces OH•-induced K+ efflux,17 a similar effect to that we reported after salt application to barley roots19 and also observed in Arabidopsis (Fig. 1). Interestingly, we found that not only known free-radical scavenging osmolytes,24 but also glycine betaine, previously found to be non-effective in ROS scavenging,24 were effective in reducing OH•-induced K+ efflux. Indeed, glycine betaine showed a greater mitigation of OH•-induced K+ efflux compared to that induced by 50 mM NaCl (Fig. 1). However, it is open to speculation as to whether this mitigation is via direct channel blocking, a direct protection of ion channel proteins or by some other protective mechanism.
Figure 1.
Effects of exogenous supply of compatible solutes on net peak K+ efflux after application of either 1 mM Cu/a or 50 mM NaCl. Roots were preincubated for 1 h in 5 mM concentration of a number of compatible solutes prior to treatment, Mean ± SE (n = 6-8).
In our further investigations we have found that salt-tolerant barley show a reduced ROS-induced K+ efflux compared to sensitive varieties.25 This superior ability of salt-tolerant barley cultivars of preventing K+ loss further indicates a possible causal link between salt and oxidative stress tolerance. We propose that upon the imposition of salt stress, the instantaneously resulting membrane depolarisation9 results in activation of depolarisation activated K+ outward-rectifying channels, leading to the initial massive K+ efflux. Over the longer term, ROS levels within the plant cell increase,7 resulting in direct damage to K+ transporters and the longer-term sustained loss of K+ from the cell. Due to mitigation of both NaCl- and OH•-induced K+ efflux by compatible solutes, we propose that one of their primary amelioratory effects is through reducing the damaging effects of salt-produced ROS on K+ transporter, and by this means, reducing the effects of stress damage. Whether this amelioration is achieved through free-radical scavenging or due to a direct protection of membrane transports warrants further investigation.
Acknowledgements
This work was supported by ARC Discovery grant (DP0449856) to S. Shabala.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/4966
References
- 1.Foyer CH, Harbinson JC. Oxygen metabolism and the regulation of photosynthetic electron transport. In: Foyer CH, Mullineaux PM, editors. Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants. Boca Rato: CRC Press; 1994. pp. 1–42. [Google Scholar]
- 2.Alscher RG, Donahue JL, Cramer CL. Reactive oxygen species and antioxidants: Relationships in green cells. Physiol Plant. 1997;100:224–233. [Google Scholar]
- 3.Pitzschke A, Forzani C, Hirt H. Reactive oxygen species signaling in plants. Antiox Red Signal. 2006;8:1757–1764. doi: 10.1089/ars.2006.8.1757. [DOI] [PubMed] [Google Scholar]
- 4.Santos CLV, Campos A, Azevedo H, Caldeira G. In situ and in vitro senescence induced by KCl stress: Nutritional imbalance, lipid peroxidation and antioxidant metabolism. J Exp Bot. 2001;52:351–360. [PubMed] [Google Scholar]
- 5.Lee SH, Singh AP, Chung GC. Rapid accumulation of hydrogen peroxide in cucumber roots due to exposure to low temperature appears to mediate decreases in water transport. J Exp Bot. 2004;55:1733–1741. doi: 10.1093/jxb/erh189. [DOI] [PubMed] [Google Scholar]
- 6.Zhu JK. Plant salt tolerance. Trends Plant Sci. 2001;6:66–71. doi: 10.1016/s1360-1385(00)01838-0. [DOI] [PubMed] [Google Scholar]
- 7.Leshem Y, Melamed-Book N, Cagnac O, Ronen G, Nishri Y, Solomon Cohen G, Levine A. Suppression of Arabidopsis vesicle-SNARE expression inhibited fusion of H2O2-containing vesicles with tonoplast and increased salt tolerance. Proc Nat Acad Sci USA. 2006;103:18008–18013. doi: 10.1073/pnas.0604421103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shabala S, Shabala L, Van Volkenburgh E. Effect of calcium on root development and root ion fluxes in salinised barley seedlings. Funct Plant Biol. 2003;30:507–514. doi: 10.1071/FP03016. [DOI] [PubMed] [Google Scholar]
- 10.Shabala L, Cuin TA, Newman I, Shabala S. Salinity-induced ion flux patterns from the excised roots of Arabidopsis sos mutants. Planta. 2005;222:1041–1050. doi: 10.1007/s00425-005-0074-2. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala S. Screening plants for salt tolerance by measuring K+ flux: A case study for barley. Plant Cell Environ. 2005;28:1230–1246. [Google Scholar]
- 12.Chen Z, Zhou M, Newman I, Mendham N, Zhang G, Shabala S. Potassium and sodium relations in salinised barley tissues as a basis of differential salt tolerance. Funct Plant Biol. 2007;34:150–162. doi: 10.1071/FP06237. [DOI] [PubMed] [Google Scholar]
- 13.Carden DE, Walker DJ, Flowers TJ, Miller AJ. Single-cell measurements of the contributions of cytosolic Na+ and K+ to salt tolerance. Plant Physiol. 2003;131:676–683. doi: 10.1104/pp.011445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuin TA, Miller AJ, Laurie SA, Leigh RA. Potassium activities in cell compartments of salt-grown barley leaves. J Exp Bot. 2003;54:657–661. doi: 10.1093/jxb/erg072. [DOI] [PubMed] [Google Scholar]
- 15.Maathuis FJM, Amtmann A. K+ nutrition and Na+ toxicity: The basis of cellular K+/Na+ ratios. Ann Bot. 1999;84:123–133. [Google Scholar]
- 16.Demidchik V, Shabala SN, Coutts KB, Tester MA, Davies JM. Free oxygen radicals regulate plasma membrane Ca2+ and K+- permeable channels in plant root cells. J Cell Sci. 2003;116:81–88. doi: 10.1242/jcs.00201. [DOI] [PubMed] [Google Scholar]
- 17.Cuin TA, Shabala S. Compatible solutes reduce ROS-induced potassium efflux in Arabidopsis roots. Plant Cell Env. 2007;30:875–885. doi: 10.1111/j.1365-3040.2007.01674.x. [DOI] [PubMed] [Google Scholar]
- 18.Köhler B, Hills A, Blatt MR. Control of guard cell ion channels by hydrogen peroxide and abscisic acid indicates their action through alternate signalling pathways. Plant Physiol. 2003;131:385–388. doi: 10.1104/pp.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuin TA, Shabala S. Exogenously supplied compatible solutes rapidly ameliorate NaCl-induced potassium efflux from barley roots. Plant Cell Physiol. 2005;46:1924–1933. doi: 10.1093/pcp/pci205. [DOI] [PubMed] [Google Scholar]
- 20.Cuin TA, Shabala S. Amino acids regulate salinity-induced potassium efflux in barley root epidermis. Planta. 2007;225:753–761. doi: 10.1007/s00425-006-0386-x. [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ. Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:463–499. doi: 10.1146/annurev.arplant.51.1.463. [DOI] [PubMed] [Google Scholar]
- 22.Holmström KO, Somersalo S, Mandal A, Palva TE, Welin B. Improved tolerance to salinity and low temperature in transgenic tobacco producing glycine betaine. J Exp Bot. 2000;51:177–185. doi: 10.1093/jexbot/51.343.177. [DOI] [PubMed] [Google Scholar]
- 23.Sakamoto A, Murata N. Genetic engineering of glycine betaine synthesis in plants: Current status and implications for enhancement of stress tolerance. J Exp Bot. 2000;51:81–88. [PubMed] [Google Scholar]
- 24.Smirnoff N, Cumbes QJ. Hydroxyl radical scavenging activity of compatible solutes. Phytochem. 1989;28:1057–1060. [Google Scholar]
- 25.Chen Z, Cuin TA, Zhou M, Naidu BP, Shabala S. Compatible solute accumulation and stress mitigating effects in barley genotypes contrasting in their salt tolerance. J Exp Bot. 2007 doi: 10.1093/jxb/erm284. In press. [DOI] [PubMed] [Google Scholar]