Abstract
Flaming a tomato leaf evokes a variation potential; excising an unwounded leaf evokes an action potential; while excising a wounded leaf 90 sec after flame-wounding evokes an action potential superimposed on the variation potential. Furthermore, flaming one leaf induces rapid (15 min), systemic and biphasic accumulation of CMBP transcript, excising the unwounded leaf causes slower, monophasic transcript accumulation, while excising the wounded leaf after 90 sec has no effect on CMBP transcript accumulation in response to the flame-wound. We propose that both of these electrical signals, the flame-evoked variation potential and the cut-wound evoked action potential are capable of inducing CMBP transcript accumulation, although with somewhat different kinetics. Earlier work by others found the cut-wound had no effect on pin transcript accumulation, thus leaf excision could be used as a tool to determine whether transport of wound hormones out of the leaf could trigger pin gene expression. Here, however, leaf excision could not be used to prevent signal transmission, since excision itself evoked an electrical signal and transcript accumulation. Instead, the results show that two different electrical signals are involved in rapid, systemic CMBP mRNA accumulation and their effects are not additive implying they may share some common aspects.
Key Words: action potential, signal transmission, tomato, variation potential, wounding
Introduction
Plants are able to sense external stimuli and transduce them into signals1 which are transmitted throughout the plant to distant, nonstimulated organs, where changes are observed in their morphology and metabolism, including gene expression.2 The tomato plant is the model system for wound-induced gene expression which involves the local and systemic induction of the proteinase inhibitor (pin) genes by stimuli such as wounding,3–5 application of various chemicals,6,7 or electrical stimulation.8,9 These genes can be induced systemically (within 6 h) by the application of chemicals such as ABA,6 oligosaccharides7 or the peptide systemin.10 They can be induced more rapidly (15–60 min) by either a flame stimulation, which triggers a variation potential (VP), or by low voltage direct current electrical stimulation, which triggers an action potential (AP), as demonstrated by Stankovic and Davies.8,9 However, there is no evidence to date to suggest that the AP and VP might be part of a larger signaling network in tomato, able to trigger the expression of genes other than proteinase inhibitors, although a strong correlation was found between changes in membrane potential and calmodulin mRNA accumulation in Bidens pilosa after both injurious and non-injurious stimulation.11
In order to determine whether there is a large array of genes up-regulated by the VP (flame-wounding), we constructed a subtractive (flamed minus control) cDNA library and identified a chloroplast mRNA binding protein (CMBP) transcript that accumulates strongly and rapidly after wounding.12 This accumulation occurs not only at the site of stimulation (i.e., the 3rd, sub-terminal leaf, but also in the distant, intact 4th terminal leaf.12
The main goal of this paper was to investigate the rapidity with which the wound signal(s) was (were) generated and transmitted, and to determine whether a chemical was likely to be the carrier of this information. Here we tested whether the removal of the damaged leaf shortly (90 s) after the flame-stimulus would prevent the transmission of the wound signal to the rest of the plant. This was one of the methods employed successfully to show that pin expression was not triggered by a transported wound hormone.13
Materials and Methods
Tomato plants (Lycopersicon esculentum cv Heinz 1439 VF) were cultivated in the North Carolina State University Phytotron under controlled conditions (L:D 16:8, 26:21°C, 300 µmol s−1m−2 light intensity) in a medium composed of gravel and Peat-lite. Three-week-old plants (about 12 cm in height) were flamed for 2 s about 1 cm below the 3rd leaf using a gas cigarette lighter (the flame set about 1.5 cm high). The stimulated leaf was either allowed to remain attached to the plant or removed by a sharp cut with a razor blade after 90 s, and the terminal leaf was collected at various times (5 to 60 min) after treatment. We used control (cut-wound) plants with the 3rd leaf excised without prior flame treatment to test the traumatic effect of leaf removal by the sharp cut. Nucleic acid isolation and characterization were performed as described earlier using the general guidelines of Sambrook et al.14 Briefly, total RNA was isolated using Trizol reagent (Gibco-BRL), separated on a formaldehyde denaturing gel and transferred onto a nylon membrane (Schleicher & Schuell). Fifty nanograms of CMBP cDNA (AF106660) was labeled with the Ready-to-Go DNA labeling system (Pharmacia) with [α-32P]dCTP, purified from unincorporated nucleotides and heat denatured. Hybridization was performed at 42°C in hybridization buffer (6× SSPE, 50% [v/v] formamide, 5× Denhardt's solution, 0.5% [w/v] SDS, and 100 mg/ml denatured herring-sperm DNA). The washes (15 min each) were performed with SSPE buffer (2× and 0.1×) containing 0.1% (w/v) SDS. The membranes were then air-dried, and autoradiographed (Fuji X-Ray films).
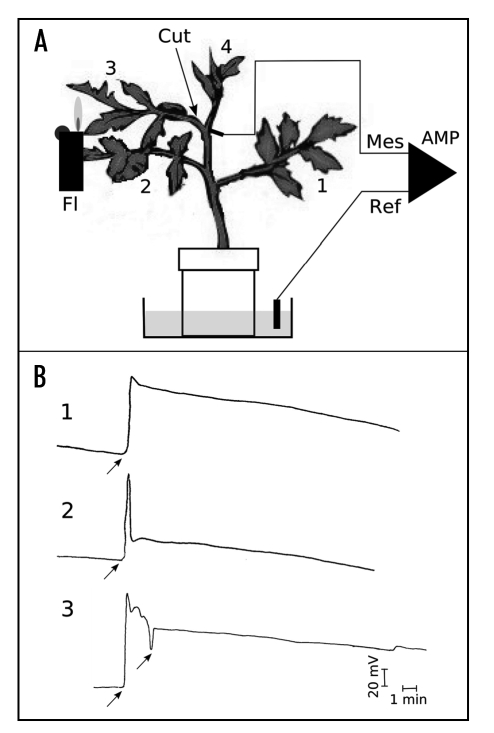
Electrophysiological measurements were performed in a Faraday cage, as described for tomato.15 Silver electrodes were inserted at the base of the petiole of the third leaf, and connected to a high impedance amplifier (World Precision Instruments KS-700, Sarasota, Florida, USA). The electric circuit was closed with a calomel reference electrode connected to the pot in the culture medium (see Fig. 1A).
Figure 1.
Plant system, treatments and electrical responses. (A) Plant set-up. Three-week-old tomato plants were placed in a Faraday cage to prevent electrical perturbation, and silver electrodes inserted in the base of the petiole of the 3rd leaf (Mes: measuring electrode) while the reference calomel electrode (Ref) was put in the culture pot. These electrodes were connected to a high impedance amplifier (Amp) and the signals recorded on a chart recorder. 1 to 4, plant leaf from the older (1) to the youngest (4). (B) Electrical responses. The plants were either: 1, flamed (Fl) on the next-to youngest 3rd leaf (3) with a 1.5 s pulse from a butane lighter; 2, cut (excised) at the base of petiole #3; or 3, flamed, and then 90 seconds later leaf #3 was excised. The vertical bar represents 20 mV and the horizontal bar 1 min.
Results
Plants were stimulated by flaming the 3rd leaf with a gas lighter for 2 s and/or excising the petiole, and the ensuing electrical responses were measured (Fig. 1B). The flame treatment evoked a typical variation potential (VP - Fig. 1B, 1) with an amplitude of about 74 mV, followed by a slow repolarization of the membrane. Cutting the petiole evoked what appeared to be an action potential (AP), with a sharp peak (77 mV in amplitude), followed by a rapid repolarization (Figs. 1B, 2). Flaming the 3rd leaf and then cutting it off at the petiole 90 s later evoked a VP similar to that seen in Figure 1A, 1, but superimposed with a sharp peak evoked by the cut (Fig. 1B, 3). The fact that the flame-induced VP and cut-induced AP could be seen simultaneously (Fig. 1B, 3) suggests that they are motivated by different channels.
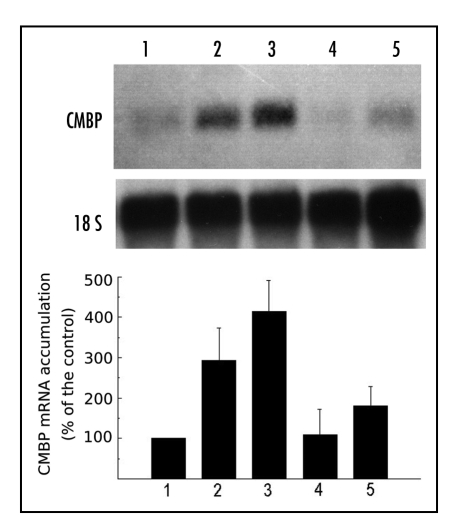
Figure 2.
CMBP mRNA accumulates systemically in leaf #4 in response to flaming leaf #3. Plants similar to those used for Figure 1 were either left intact (control, lane 1), or flame-wounded on leaf #3, and leaf #4 was harvested after 5 min (lane 2), 15 min (lane 3), 30 min (lane 4) or 60 min (lane 5) and used for RNA isolation and Northern blots. CMBP, chloroplast mRNA binding protein, and 18S rRNA as the control for gel loading. Graph showing expression of CMBP relative to unflamed plant taken as base 100 reference.
Flame treatment of the 3rd leaf caused a rapid accumulation of CMBP transcript in the distant, intact terminal 4th leaf (Fig. 2) as seen by direct examination of the blots (Fig. 2A) as well as calculations of the CMBP:rRNA ratio (Fig. 2). The steady state level was low in control plants (Fig. 2 lane 1), but within 5 min after treatment, a 2- to 3-fold increase in transcript level had already occurred (Fig. 2 lane 2), was maximal (about 4-fold) by 15 min (Fig. 2, lane 3), rapidly dropped to that observed in control plants by 30 min (Fig. 2, lane 4) before increasing again at 60 min (Fig. 2, lane 5).
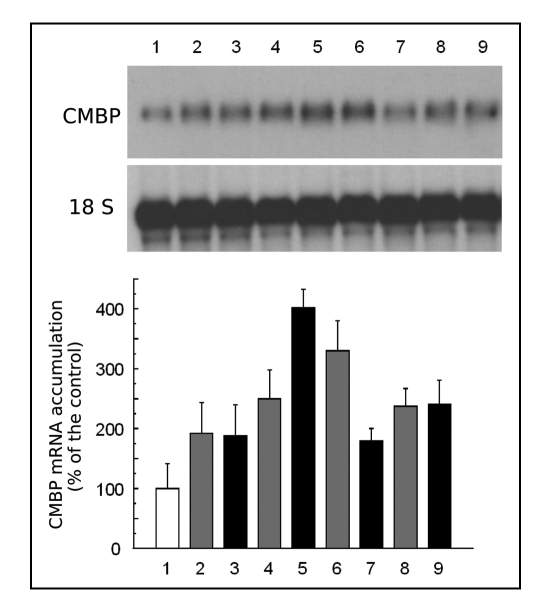
In order to understand the underlying mechanism behind the rapid transmission of the flame signal, we repeated the experiment, but removed the flamed leaf 90s after the stimulus. Because the removal of the petiole itself constituted a wound, we made control (named “cut only”) samples for each time point to evaluate the effects of cutting on CMBP transcript accumulation (Fig. 3). As in (Fig. 3) (lane 1), the steady state level was low in control plants (Fig. 3, lane 1) while the sharp cut of the petiole evoked a signal (Fig. 1B) capable of causing transcript accumulation in the 4th leaf to the same extent (Fig. 3, lane 2) as the combination of flame followed by a cut (Fig. 3, lane 3). However, by 15 min after treatment the response to the two stimuli was different. The petiole cut caused a slight increase in CMBP transcript accumulation (Fig. 3, lane 4), but the prior flame treatment caused a massive increase (Fig. 3, lane 5) similar in magnitude to that observed after the flame treatment alone (Fig. 2). In tissue subjected to the flame-wound followed by petiole excision, the CMBP transcript level decreased at 30 min (Fig. 3, lane 7) before accumulating again (Fig. 3, lane 9) similar to tissue subjected to the flame-wound alone (Fig. 2, lanes 3 and 4). Interestingly, transcript levels continued to rise at 30 min in the tissue subjected to the cut alone (Fig. 3, lane 6), but declined at 60 min (Fig. 3, lane 8).
Figure 3.
Excision of the flamed leaf (#3) has little effect on systemic CMBP mRNA accumulation in leaf #4. Plants similar to those used in Figure 1 were either left untreated (control, lane 1, white box), cut-wounded by excision of leaf #3 (grey boxes) and leaf #4 harvested after 5 min (lane 2); 15 min (lane 4); 30 min (lane 6) or 60 min (lane 8); or flame-wounded on leaf #3 and then leaf #3 excised 90 sec later (black boxes) and leaf #4 harvested after 5 min (lane 3), 15 min (lane 5), 30 min (lane 7) or 60 min (lane 9) and assayed for CMBP mRNA and 18S rRNA. Graph showing expression of CMBP relative to unflamed plant taken as base 100 reference.
Discussion
Systemic accumulation of stress-related transcripts after various stimuli is a well-described phenomenon in tomato,16 especially for the pin genes, but the nature of the wound signal(s) rapidly transmitted throughout the plant is still in debate. The major candidate is a molecular (hormonal) signal. In support of this view, the work of Ryan and Farmer and Peña-Cortès et al.17,18 demonstrates that several hormones, including oligosaccharides, ABA and systemin, switch on pin gene expression systemically, although accumulation of the pin transcript is typically measured 6 h after stimulus. These results clearly and undoubtedly demonstrate the involvement of a molecular carrier in some aspect of long-term (6 h) pin mRNA accumulation in tomato, although the role of ABA has been questioned,19,20 A second candidate is an electrical signal21 (AP), which was implicated by the work of Wildon et al.13 who showed that for short term responses (60 min), transmitted changes in membrane potential could be related to pin gene expression. The role of electrical signals (APs) was further supported by Stankovic and Davies8,9 who conducted experiments on more rapid (15–30 min) systemic pin transcript accumulation in tomato and concluded that a strict relationship exists between pin mRNA accumulation and the transmission of membrane depolarization to the analyzed tissues. These authors proposed a third candidate for the wound signal evoking pin transcript accumulation as being a transmitted hydraulic signal (loss of tension in the xylem) with a local electrical aftermath (VP) in addition to the genuine electrical signal (AP). An alternative explanation involving the rapid transmission of a premade chemical in the surging xylem has been offered (Malone et al.22 and Malone23).
It is not known if these phenomena are restricted to the pin gene family in tomato, or whether such electrical-based signaling systems evoke the expression of a wide array of genes. It seems likely not to be restricted to the pin gene family, since similar observations on the role of membrane depolarization and accumulation of calmodulin transcript have been described after flaming in the composite Bidens pilosa.11
Using a subtractive cDNA library (flame minus control), we isolated a novel wound-induced clone, encoding a chloroplast mRNA binding protein whose transcript accumulates strongly and systemically after flaming in tomato.12 Here we show that flame-wounding evokes a typical VP (Fig. 1B) as has been described previously in tomato, while cutting alone causes the generation and transmission of an AP (Fig. 1B), similar to that evoked by electrical stimulation.8 Cutting the leaf 90 sec after the flame-wound induced an AP superimposed on the VP (Fig. 1B). Analysis of CMBP transcript levels showed that flame-wounding alone caused rapid, systemic transcript accumulation beginning in 5 min, with a peak at 15 min, a decline, and a second increase at 60 min (Fig. 2). This is very similar to the biphasic response seen earlier.12 Leaf excision (cut-wound), the technique that we had intended to use as a method to prevent passage of a chemical signal (wound hormone) as had been done earlier for the pin transcript13 also caused CMBP transcript accumulation. Transcript accumulation was equally rapid initially (5 min), but with a peak at 30 min (Fig. 3) instead of 15 min as seen with flame-wounding (Fig. 2). Because excision of leaf #3 induced systemic CMBP transcript accumulation in leaf #4, it obviously could not be used as a system for preventing transport of a putative chemical signal evoking CMBP mRNA accumulation. Instead we were able to use it as a means of demonstrating that AP evoked by a cut-wound (Fig. 3) caused CMBP mRNA accumulation. This is very similar to the earlier finding that AP generated in response to electrical stimulus cause systemic pin mRNA accumulation,8,9 except that here we show that transcript accumulation occurred within 5 min (Fig. 3) rather than the 15 min as shown earlier. Although we could not use excision of the flamed leaf as a method to try to prevent transmission of a wound chemical, we did use the method to show that there was no additive or synergistic effect between transcript accumulation in response to flaming (Fig. 2) and to cutting (Fig. 3). In fact, the effect of the cut (leaf excision after the flame-wound) was negligible - the response to flame plus cut (Fig. 3) was very similar to the response to flame alone (Fig. 2).
In trying to untangle the complexity of many systemic plant responses to the environment, we have suggested,24,25 that there is a series of stimulus/signal events, namely:
“External stimulus → transmitted signal (= local stimulus) → signal (= stimulus or second messenger) → response”,
where the initial stimulus (here a flame or cut) evokes a transmitted signal (AP, VP or hormone), which in turn acts in distant tissue as a local stimulus to evoke another signal (perhaps calcium) which becomes a stimulus for another signal (perhaps InsP3) with some eventual response (here transcript accumulation). Based on this hypothetical pathway,25 we can use the results here to argue for and against certain putative explanations. First, flame-wounding and cutting are almost equally effective in causing transcript accumulation, yet they have no additive effect. This strongly implies that there must be some common (limiting) point in the respective pathways. The fact that they evoke different electrical signals (VP and AP) says that the systemic signal is not the common point. Further, since VP-evoked calcium26 appears to enter through mechano-sensitive channels in living cells close to the xylem, whereas AP-evoked calcium enters voltage-gated channels in the phloem,27 the common point may not be calcium. It is more likely that InsP3 is the common point, since any increase in intra-cellular calcium is likely to stimulate an increase in InsP3.28 However, the rate of transcription by RNA polymerase II (pol2), whether evoked by calcium and/or InsP3, is perhaps the most likely common point, especially considering that pol2 activity is enhanced by phosphorylation,29 which, in turn, is normally modulated by calcium.
Acknowledgements
The authors wish to thank the North Carolina Agricultural Research Service (grant #06446), the NCSU Phytotron for growing the tomato plants and the National Aeronautic and Space Administration (grant # 547-574) for supporting this research. Thanks to J.L. Julien (Blaise Pascal University) for advice on electrophysiology.
Abbreviations
- AP
action potential
- CMBP
chloroplast mRNA binding protein
- InsP3
inositol-1,4,5-trisphosphate
- pin
proteinase inhibitor
- VP
variation potential
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=3325
References
- 1.Davies E, Schuster A. Intercellular communication in plants: Evidence for a rapidly generated, bidirectionally transmitted wound-signal. Proc Natl Acad Sci USA. 1981;78:2422–2426. doi: 10.1073/pnas.78.4.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reymonda P, Webera H, Damonda M, Farmer EE. Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell. 2000;12:707–720. doi: 10.1105/tpc.12.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham JS, Pearce G, Merryweather J, Titani K, Ericsson L, Ryan CA. Wound-induced proteinase inhibitors from tomato leaves. II. The cDNA-deduced primary structure of preinhibitor II. J Biol Chem. 1985;260:6561–6564. [PubMed] [Google Scholar]
- 4.Herde O, Artzorn R, Fisahn J, Wasternack C, Willmitzer L, Peña-Cortès H. Localized wounding by heat initiates the accumulation of proteinase inhibitor II in abscisic acid-deficient plants by triggering jasmonic acid biosynthesis. Plant Physiol. 1996;112:853–860. doi: 10.1104/pp.112.2.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herde O, Peña-Cortès H, Willmitzer L, Fisahn J. Time-resolved analysis of signals involved in systemic induction of pin-2 gene expression. Bot Acta. 1998;111:383–389. [Google Scholar]
- 6.Peña-Cortès H, Willmitzer L, Sanchez-Serrano JJ. Abscisic acid mediates wound induction but not developmental-specific expression of the proteinase inhibitor II gene familiy. Plant Cell. 1991;3:963–972. doi: 10.1105/tpc.3.9.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan CA, Farmer EE. Oligosaccharide signals in plants: A current assessment. Plant Physiol Plant Mol Biol. 1991;42:651–674. [Google Scholar]
- 8.Stankovic B, Davies E. Both action potentials and variation potentials induce proteinase inhibitor gene expression in tomato. FEBS Lett. 1996;390:275–279. doi: 10.1016/0014-5793(96)00672-2. [DOI] [PubMed] [Google Scholar]
- 9.Stankovic B, Davies E. Intercellular communication in plants: Electrical stimulation of proteinase inhibitor gene expression in tomato. Planta. 1997;202:402–406. [Google Scholar]
- 10.Pearce G, Strydom D, Johnson S, Ryan CA. A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science. 1991;253:895–898. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- 11.Vian A, Henry-Vian C, Schantz R, Ledoigt G, Frachisse JM, Desbiez MO, Julien JL. Is membrane potential involved in calmodulin gene expression after external stimulation in plants? FEBS Lett. 1996;380:93–96. doi: 10.1016/0014-5793(96)00015-4. [DOI] [PubMed] [Google Scholar]
- 12.Vian A, Henry-Vian C, Davies E. Rapid and systemic accumulation of chloroplast mRNA binding protein transcripts after flame stimulus in tomato. Plant Physiol. 1999;121:517–524. doi: 10.1104/pp.121.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wildon DC, Thain JF, Michin PEH, Gubb IR, Reilly AJ, Skipper YD, Doherty HM, O'Donnel PJ, Bowles DJ. Electrical signaling and systemic proteinase inhibitor induction in the wounded plant. Nature. 1992;360:62–65. [Google Scholar]
- 14.Sambrook J, Fritsch EF, Maniatis T. In: Molecular cloning: A laboratory manual. Second edition. Nolan C, editor. New York: Cold Spring Harbor Laboratory Press; 1989. (Chapter 7) [Google Scholar]
- 15.Stankovic B, Davies E. The wound response in tomato involves rapid growth and electrical responses, systemically up-regulated transcription of proteinase inhibitor and calmodulin and down-regulated translation. Plant and Cell Physiol. 1998;39:268–274. [Google Scholar]
- 16.Cheong YH, Chang HS, Gupta R, Wang X, Zhu T, Luan S. Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in arabidopsis. Plant Physiol. 2002;129:661–677. doi: 10.1104/pp.002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peña-Cortès H, Fisahn J, Wilmitzer L. Signals involved in wound-induced proteinase inhibitor II gene expression in tomato and potato plants. Proc Natl Acad Sci USA. 1995;92:4106–4113. doi: 10.1073/pnas.92.10.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peña-Cortès H, Prat S, Artzon R, Wasternack C, Willmitzer L. Abscisic-acid deficient plants do not accumulates proteinase inhibitor II following systemin treatment. Planta. 1996;198:447–451. [Google Scholar]
- 19.Birkenmeier GF, Ryan CA. Wound signaling in tomato plants. Plant Physiol. 1998;117:687–693. doi: 10.1104/pp.117.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herde O, Peña-Cortès H, Wasternack C, Willmitzer L, Fisahn J. Electric signaling and pin2 gene expression on different abiotic stimuli depend on a distinct threshold level of endogenous abscisic acid in several abscisic acid-deficient tomato mutants. Plant Physiol. 1999;119:213–218. doi: 10.1104/pp.119.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies E. Action potentials as multifunctional signals in plants: An unifying hypothesis to explain apparently disparate wound responses. Plant Cell Environ. 1987;10:623–631. [Google Scholar]
- 22.Malone M, Alarcon JJ, Palumbo L. An hydraulic interpretation of rapid, long-distance wound signaling in tomato. Planta. 1994;193:181–185. [Google Scholar]
- 23.Malone M. Rapid, long-distance signal transmission in higher plants. Adv Bot Res. 1996;22:163–228. [Google Scholar]
- 24.Davies E. Electrical signals in plants: Facts and hypotheses. In: Volkov AG, editor. Plant electrophysiology - Theory and Methods. Berlin: Springer-Verlag; 2006. pp. 407–422. [Google Scholar]
- 25.Davies E, Stankovic B. Electrical signals, nnnthe cytoskeleton, and gene expression: Current hypotheses. In: Baluska F, Mancuso S, Volkmann D, editors. Communication in Plants - Neuronal Aspects of Plant Life. Berlin: Springer-Verlag; 2006. pp. 309–320. [Google Scholar]
- 26.Fisahn J, Herde O, Willmitzer L, Peña-Cortès H. Analysis of the transient increase in cytosolic Ca2+ during the action potential of higher plants with temporal resolution: Requirement of Ca2+ transients for induction of jasmonic acid biosynthesis and PINII gene expression. Plant and Cell physiol. 2004;45:456–459. doi: 10.1093/pcp/pch054. [DOI] [PubMed] [Google Scholar]
- 27.Davies E. Intercellular and intracellular signals and their transduction via the plasma membrane-cytoskeleton interface. Cell Biol. 1993;4:139–147. doi: 10.1006/scel.1993.1017. [DOI] [PubMed] [Google Scholar]
- 28.Perera IY, Hung CY, Brady S, Muday GK, Boss WF. A universal role for inositol 1,4,5-trisphosphate-mediated signaling in plant gravitropism. Plant Physiol. 2006;140:746–760. doi: 10.1104/pp.105.075119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landick R. Shifting RNA Polymerase into Overdrive. Science. 1999;284:598–599. doi: 10.1126/science.284.5414.598. [DOI] [PubMed] [Google Scholar]