Abstract
The aim of this study was to investigate the influence of sugars on blue light-induced chloroplast movements. Sucrose and glucose inhibited chloroplast responses in the detached leaves of Arabidopsis thaliana and in Lemna trisulca fronds in a concentration and time-dependent manner. The prolonged exposure necessary for inhibition indicates that sugars may act via altered gene expression. Overexpression of phototropin2, a photoreceptor responsible for the strong blue light response of chloroplasts, counteracted the sugar effect. This may suggest that sugars modify some component(s) of the phototropin2-mediated signal transduction pathway. The expression of PHOT2 was not suppressed by sugars in wild type plants, it was even upregulated by glucose. Impaired chloroplast movements were observed only in mature Arabidopsis plants. The mRNA of SAG12, a late senescence marker, was not detectable in the sugar-incubated leaves. The SAG13 mRNA level and its regulation by sugars were similar in wild type and PHOT2 overexpressor. Thus, the sugar insensitivity of 35S:PHOT2 chloroplast responses was not due to delayed senescence. The sugar-induced transduction pathway involved remains unclear. 3-O-methylglucose did not affect chloroplast movements suggesting the participation of a hexokinase-dependent pathway. Only the amplitude of avoidance response was reduced in gin2-1, a hexokinase1 null mutant. Probably other hexokinases, or glycolysis-associated signals play a role in the suppression of chloroplast responses.
Key Words: Arabidopsis thaliana, blue light, chloroplast movements, glucose, hexokinase, Lemna trisulca, mannose, phototropin, signaling, sucrose
Introduction
Plants as sessile organisms must possess mechanisms allowing them to cope with changes in their environment. Light not only supplies energy for photosynthesis, but also regulates the expression of photosynthetic genes, acts as a switch between skoto- and photomorphogenesis, and stimulates movement responses. To optimize the light dose plants can grow towards or away from a light source, and change the position of their organs. In many plant species chloroplasts move directionally inside the cell. These rearrangements are precisely defined by the intensity, spectrum and direction of light.1 Strong light causes chloroplast displacement to the anticlinal walls (the avoidance response), whereas in weak light they gather along periclinal walls (the accumulation response). The former response helps to prevent the photodestruction of the photosynthetic apparatus by excess light, the latter being responsible for optimal photosynthesis.2 Chloroplast relocations can be induced by blue and red light in some algae and ferns; in angiosperms, like Arabidopsis thaliana, Lemna trisulca and Nicotiana tabacum, they are activated by blue light only. The photoreceptors responsible for chloroplast translocations in blue light are phototropins (phot1 and phot2). The activity areas of these two photoreceptors partially overlap, as observed in phototropism, guard cell and chloroplast movements.3,4 In the latter process phot1 responds to a broad range of fluence rates by initiating the accumulation response only, whereas phot2 activates both responses in dependence on fluence rate.5 The avoidance response, controlled by phot2, is initiated at about 20 “mol m−2 s−1 of blue light and saturated at about 120 µmol m−2 s−1. In mutant plants lacking phot2 both strong and weak blue light activate the accumulation response. Knowledge of the regulation of the expression of phototropins, the stability of proteins and their turnover is still incomplete. Most experiments have been done with Arabidopsis seedlings and no data on mature leaves are available. Whereas white light, blue light and UV-A induce the expression of PHOT2 in Arabidopsis thaliana etiolated seedlings,5 white light downregulates the expression of PHOT1,6 as measured at the mRNA level. Interestingly, the amount of protein reacting with an anti-phot1 antibody remains almost the same in oat coleoptiles and cress hypocotyls both in the dark and after blue-light illumination.7
Similarly to light, the substrate for photosynthesis, its product sugars regulate the expression of many genes and the activities of enzymes involved in photosynthesis, glycolysis, glyoxylate metabolism, nitrogen, sucrose and starch metabolism (for review see refs. 8 and 9). Sugars also affect cell cycle regulation,10 seed development, germination and senescence.11
Sucrose, the common transportable sugar and other disaccharides as well as hexoses act as signal molecules using different signal transduction pathways. As photosynthetic organisms, plants must be able to distinguish between sugars produced inside the cell and those transported from the apoplast. Cells sense changes in the concentration of sugar, not its quantity per se. Thus, it has been proposed that the transport of sugar through membranes is required to induce signaling.12 Actually, in some cases even membrane non-permeable sugars (for example non-transportable sucrose analogs like turanose and palatinose) act as signal molecules, indicating the involvement of cell surface receptors.13 Abundant evidence exists that hexokinase plays a pivotal role in sugar sensing in plant cells, as it does in yeast and mammals. Arabidopsis hexokinases are predicted to be membrane bound,11 which is compatible with the postulated role of membrane flux in signal initiation. At least three different hexose signal transduction pathways are present in plants: (1) hexokinase dependent, (2) glycolysis dependent, (3) hexokinase independent.14
Signaling networks are interconnected, and various secondary messengers and effector systems may be shared between the pathways activated by different stimuli.15 Numerous facts point to the existence of links between sugar and phosphate, nitrogen, hormone as well as light signaling pathways.9,16–18 For example the expression of many ion transporters in roots is regulated diurnally by the light/dark transition and this effect may be mimicked by sugars but not by sugar analogs.19 Likewise, the expression of photosynthesis-related genes like CABs, PC and RBCS is enhanced by light, whereas glucose treatment can override this upregulation.15 Metabolizable sugars, sucrose and glucose strongly promote the inhibitory activity of phytochrome B in the control of hypocotyl elongation by phytochrome A.20
In the present work we examined potential links between exogenously supplied sugars and the blue light-activated pathways controlling chloroplast redistribution. Two species of water and terrestrial angiosperms were investigated: Lemna trisulca and Arabidopsis thaliana. These were used as model plants in the studies on light-induced chloroplast responses in our laboratory. The starting point was the observation that sucrose added to the culture medium inhibited chloroplast redistribution in Lemna. This inhibition was strong even though sucrose promoted the growth and development of the duckweed. The availability of Arabidopsis mutants and gene sequences enabled a more detailed examination of the sugar effect.
Materials and Methods
Chemicals.
D(+) mannoheptulose was obtained from Glycoteam GmbH, Germany. Sucrose and glucose came from POCh, Poland. Other chemicals were from Sigma-Aldrich, USA.
Plant material.
Lemna trisulca L. was obtained from the collection of Jagiellonian University Botanical Garden, Krakow, Poland. Arabidopsis thaliana wild type (WT) seeds were purchased from Lehle Seeds (Round Rock, TX, USA). The seeds of PHOT2 overexpressor (35S) were the kind gift of J. Jarillo, Departamento de Biotecnología, Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria, Madrid, Spain; gin2-1 mutant seeds were obtained from NASC (stock number: N6383). Arabidopsis thaliana WT and PHOT2 overexpressor were Columbia background, gin2-1 was Landsberg erecta background.
Plant growth conditions.
Arabidopsis plants were grown in commercial soil in an environmental chamber (Sanyo MLR 350H, Japan) at 23°C with a constant 85% humidity. Illumination was provided by fluorescent lamps (Sanyo, FL40SS.W/37) with a 10 L/14 hD photoperiod (L: 1 h 50 µmol m−2 s−1, followed by 8 h of 90 µmol m−2 s−1 then by 1 h of 50 µmol m−2 s−1). Arabidopsis developed relatively slowly under these conditions and started bolting after approximately 6 weeks. Lemna was cultured in vitro in a liquid 1:5 Appenroth & Teller medium21 at the same temperature and light conditions as Arabidopsis.
Photometric method.
A double-beam custom-made photometer was used for quantitive measurements of chloroplast relocations in response to blue-light illumination.22 This method is based on the dependence between chloroplast distribution and light transmission. monochromatic red light 2 = 660 µm at a fluence-rate of 0,1 µmol m−2, modulated with a frequency of 800 Hz, served as the measuring light.
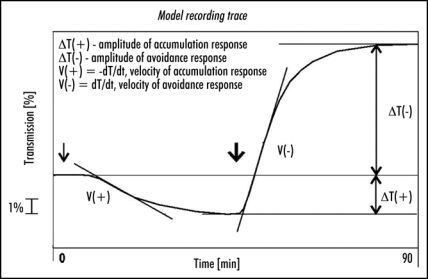
Leaves were dark-adapted for at least 14 h before each experiment. Only leaves with similar levels of transmission after dark adaptation were selected. The samples were illuminated with weak blue light (1,6 µmol m−2 s−1) for 45 min, followed by strong blue light (120 µmol m−2 s−1) for the same period. The following parameters were measured/calculated to characterize the chloroplast responses: (1) amplitudes—transmission changes after 45 min of each response referred to the initial transmission level, (2) velocities—first derivatives of the initial (lasting about 10 min) linear fragments of the respective transmission curves. The way of calculating the parameters is shown in Figure 1.
Figure 1.
A model recording trace explaining the way in which amplitudes and velocities were calculated. Arrows indicate start of illumination with weak (↓1,6 µmol m−2 s−1) and strong (↓120 µmol m−2 s−1) blue light.
Actinic light was provided by a halogen lamp (100W, 12V, Polam, Poland). A combination of filters: BG12, BG23, GG13 and a heat-absorbing C805 (all from Schott, Jena, Germany) was used to obtain blue light with a maximum emission at 423 nm and a half-band width of 86 nm. The weak light was obtained with neutral density filters.
Exposure to sugars.
Sugars were added to the medium before autoclaving. Detached Arabidopsis thaliana leaves were incubated for one or two days on 0.8% agar with/without sugar. A similar method of incubation on agar has been described by Ohto and Nakamura.23 The petioles were inserted into the medium and the leaves were pressed against agar. As gin2-1 leaves are cup-shaped, they were cut apart along the main vein a few mm from the leaf apex to ensure a proper contact with the medium. Sterile grown Lemna trisulca was exposed to sugars added to the culture medium for one or two days. The adaptation to sugars/sugar analogs started under the same light regime as the plant culture and continued in the dark for the final 14 h preceding transmission measurements. Leaves used for RNA isolation were treated in the same manner. Only healthy sixth or seventh true leaves from non-bolting Arabidopsis plants were chosen for experiments.
RNA isolation, RT-PCR and semi-quantative PCR.
Total RNA was isolated using RNeasy Plant Mini Kit (Qiagen GmbH, Germany). DNA contaminations were removed with a DNA-free™ Kit (Ambion Europe Ltd UK). First-strand cDNA synthesis was performed using a RevertAid™ First Strand cDNA Synthesis Kit (Fermentas UAB, Lithuania) and primed with random hexamer primers.
QuantumRNA™ 18S RNA (Ambion Europe Ltd UK) with 3:7 primer:competimer ratio functioned as an internal standard in the simultaneous PCR amplification with all genes except CABs. In the latter case a 1:5 dilution of cDNA was used with a 5:5 primer: competimer ratio. The following gene specific primers were used:
PHOT2 (forward: 5′-GACGCTACACAGCCTCACTGTCCC-3′; reverse: 5′-TCCCAACTGTCCCTCTGCCCTATT-3′),
MYB4 (forward: 5′-TCCGGTGGATCAACTACTCCCGG-3′; reverse: 5′-ACAACGTGGCGTTGTTGACTTTCCA-3′),
CABs (forward: 5′-AAGTACTTGGGTCCATTCTCTGG-3′, reverse: 5′-GAATCCAAACATAGAGAACATAGC-3′),
UGP (forward: 5′-AAGCTCGATACTTTCTTATCACAGG-3′, reverse: 5′-CGAGCTCGACTATACTAGGAATGG-3′),
SAG12 (forward: 5′-TGTTTCTCCATCACTCTTTCTCG-3′, reverse: 5′-TGCATGATCAAGATACGTAGTGC-3′) and SAG13 (forward: 5′-CTTGGGAGAGAACTCAAGATGG-3′, reverse: 5′-AGAGTGAACTCTTGTTCACCACG-3′).
The CAB primers were specific to genes belonging to the family of chlorophyll a/b binding proteins (i.e Lhcb1.1, Lhcb1.2, Lhcb1.3 and Lhb1b2).
Results
Time- and concentration-dependent inhibition of chloroplast relocation by sugars.
Glucose, sucrose and mannose reduced the amplitude and velocity of the accumulation and the avoidance responses in both studied species. The effect of sugars depended on their concentration and on the time of incubation. At least a day-long (and, in most cases a two-day-long) incubation was needed for any evident perturbation of chloroplast movements. Chloroplast responses were unaffected by 3% sugars applied for only a few hours (data not shown).
Lemna trisulca.
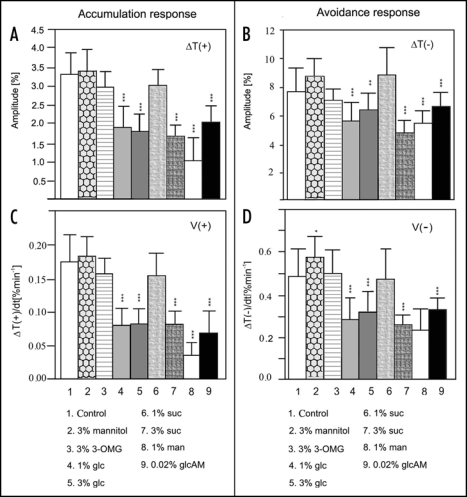
While the growth and development of duckweed were enhanced in the medium enriched in glucose and/or sucrose, chloroplast responses were markedly reduced. The inhibitory effect of glucose was much stronger than that of sucrose. A concentration of 1% of glucose present for two days in the mixotrophic culture was sufficient to strongly inhibit the movements (Fig. 2, col. 4). An increase of the glucose concentration to 3% did not change the extent of this inhibition (Fig. 2, col. 4 vs 5). Sucrose at a concentration of 1% was ineffective in Lemna (Fig. 2, col. 6) but, at a concentration of 3% it clearly reduced chloroplast responses to both strong and weak light (Fig. 2, col. 7). The extent of inhibition was similar in 3% glucose or sucrose after a two-day exposure. Weak light-induced movements were more sensitive to the sugars than those activated by strong light.
Figure 2.
Chloroplast relocations in Lemna trisulca fronds after a two-day exposure: (A) amplitudes of accumulation response induced by weak blue light (1,6 µmol m−2 s−1) (B) amplitudes of avoidance response in strong blue light (120 µmol m−2 s−1), velocities in (C) weak blue light, and (D) strong blue light. Each column represents the average of six to twelve measurements, each carried out with a different frond. Error bars represent SD. Asterisks denote the significance of differences (p-value calculated with the unpaired t-test, *p = 0,01-0,05; **p = 0,01-0,001; ***p < 0,001). 3-OMG - 3-O-methylglucose, glc - glucose, suc - sucrose, man - mannose, glcAM - glucosamine.
Mannose worked as a powerful inhibitor of all chloroplast translocations at a concentration as low as 1% (Fig. 2, col. 8). The sugar concentrations used were far too low to cause plasmolysis. Additionally, to exclude an osmotic effect Lemna fronds were treated with mannitol, a non-transportable sugar alcohol. Chloroplast relocations in weak blue light were almost unchanged in duckweed treated with mannitol (cf. Fig. 2A and C, col. 2) and those in strong light were slightly enhanced (cf. Fig. 2B and D, col. 2).
Arabidopsis thaliana wild type.
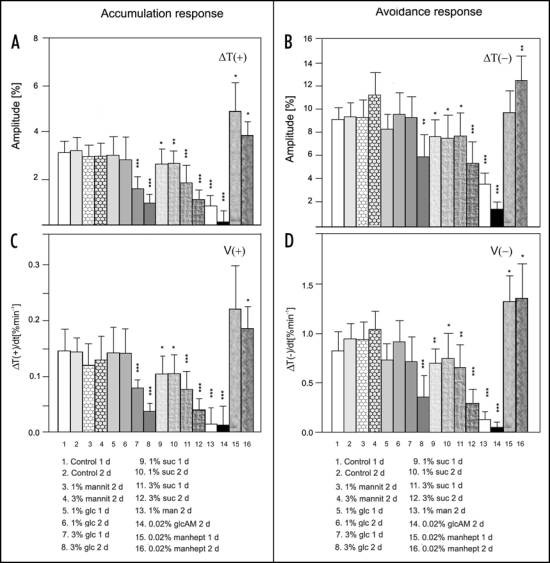
Sugar inhibition of chloroplast movements did not occur in Arabidopsis grown in vitro with sugars added to the medium. Sugars delivered via roots either did not affect or even intensified chloroplast responses, depending on light conditions during growth. Thus, sugars required a direct contact with the cell to inhibit the movements. Preliminary experiments with attached Arabidopsis leaves incubated with glucose showed a similar level of inhibition to that observed in detached leaves (unpublished results). The incubation of detached leaves on agar alone did not influence blue light-induced chloroplast responses. Neither their amplitudes nor velocities were changed in control leaves incubated for two days on agar (Fig. 3, col. 1 and 2).
Figure 3.
Influence of exposure time and sugar concentration on chloroplast responses in leaves of wild type Arabidopsis thaliana: amplitudes of (A) accumulation response and (B) avoidance response; velocities of (C) accumulation response and (D) avoidance response. The accumulation response was activated by weak blue light of 1.6 µmol m−2 s−1 and avoidance response by strong blue light of 120 µmol m−2 s−1. Each column represents the average of six to twelve measurements of individual leaves from different plants. Error bars represent SD. Asterisks denote the significance of differences (p value calculated with the unpaired t-test, *p = 0,01-0,05; **p = 0,01- 0,001; ***p < 0,001). Mannit, mannitol; mannohept, mannoheptulose; other abbreviations as in Figure 2.
In contrast to Lemna, chloroplast responses in wild type Arabidopsis were more sensitive to sucrose than to glucose: 1% glucose did not affect chloroplast movements even after two days of incubation (Fig. 3, col. 5 and 6); 3% glucose was needed to impair the movements in a time-dependent manner (Fig. 3, col. 7 and 8). The inhibitory effect of sucrose was significant at a lower concentration (1%) and at a shorter incubation time (1 day, Fig. 3, col. 9). Both sugars were equally effective at a concentration of 3% and a 2 day-long exposure. The amplitudes (Fig. 3A, col. 8 and 12) and velocities of accumulation responses (Fig. 3C, col. 8 and 12) were reduced by almost 70%, similar to Lemna (cf. Fig. 3A–D, col. 5,7). The avoidance responses were less suppressed than the accumulation ones: their amplitudes were reduced by about 40% (Fig. 3B, col. 8,12) and their velocities were reduced by half (Fig. 3D, col. 8,12).
Mannose at a concentration of 1% very strongly disturbed chloroplast relocations (Fig. 3 col. 13). However, at 0.5% it was ineffective (data not shown). Again, the accumulation response was more susceptible. Similar to Lemna, mannitol did not affect chloroplast movements in Arabidopsis mesophyll cells. Exposure to 3% mannitol for two days even slightly enhanced the chloroplast response to strong blue light, whereas the accumulation response remained unchanged (cf. Fig. 3A and B, col.4).
Interestingly, inhibition by glucose and sucrose was observed only in mature Arabidopsis plants, older than five weeks, indicating a mechanism related to a specific developmental stage. Only mannose repressed chloroplast response to continuous blue light in younger, three-to four-week old plants (data not shown). Therefore, six-week old plants were used in all reported experiments.
Other sugar effects; the expression of typical sugar-responsive genes.
Exposure to glucose and sucrose resulted in an accumulation of anthocyanins in all lines used for experiments, easily noticeable after one day and strong after two days. This typical, sugar-induced effect24 was visible in both Lemna fronds and Arabidopsis leaves. The accumulation of anthocyanins was localized along the veins, in the lower epidermis of Arabidopsis leaves. The mesophyll tissue stayed green. No anthocyanin accumulation was observed after mannose treatment: the leaves were green but they were more transparent. Incubation with all other sugars and/or sugar analogs did not produce any visible effects.
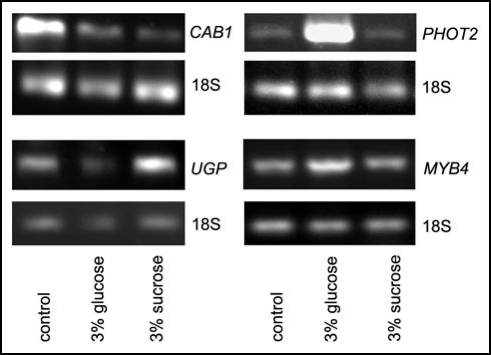
To ensure a proper penetration of sugars inside the Arabidopsis tissue, we checked the expression of several genes, CABs, UGP and MYB4, encoding respectively: chlorophyll a/b binding proteins, UDP-glucose pyrophosphorylase and a transcription factor belonging to a myb family. A reduction of transcript abundance for CABs is one of the typical effects induced by both sugars.25 Sucrose-specific induction of UGP has been demonstrated in detached Arabidopsis leaves.26 The same type of regulation has been found for MYB4 in 14 day old Arabidopsis seedlings.27,28 According to expectations, the expression of CABs was reduced in the leaves fed with 3% glucose and/or sucrose for 2 days (Fig. 4). Also UGP mRNA level increased, as expected, in the presence of sucrose. However, the expression of MYB4 was upregulated only by glucose and not by sucrose (Fig. 4).
Figure 4.
Expression of PHOT2, CABs, UGP and MYB4 in wild type Arabidopsis leaves incubated with 3% sugars for 48h after 14 h of dark adaptation. The experiment was repeated three times with similar results.
Although the Arabidopsis leaves used in the experiments came from soil-grown plants, no bacteria or fungi contamination was visible even after a two-day incubation on sugar-enriched plates. No differences in appearance were detectable between WT, gin2-1 and PHOT2 overexpressor leaves. No yellowing, a typical senescence symptom, was visible in the sugar-treated leaves of these plants.
Influence of sugars on the functioning of Phototropin2.
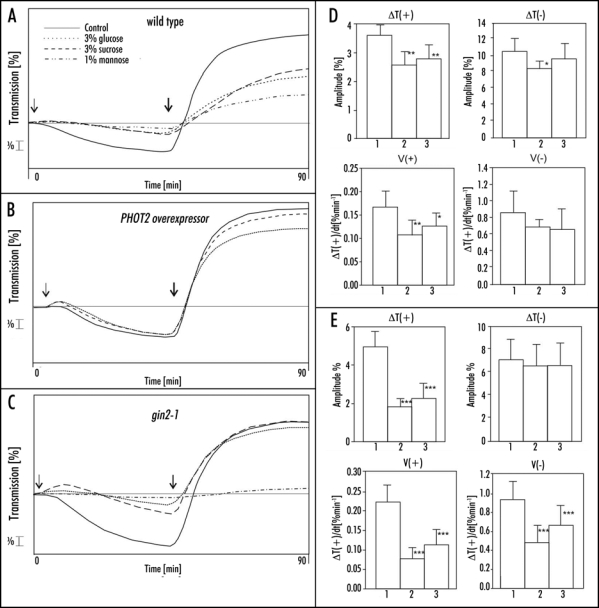
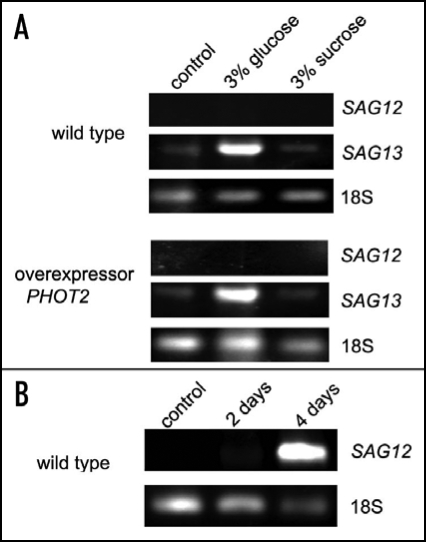
Arabidopsis PHOT2 overexpressor was used to test a potential role of phototropin2 in the sugar inhibition of chloroplast relocations. Chloroplast movements were much less affected by 3% sucrose or glucose in the overexpressor (Fig. 5B) leaves than in WT (Fig. 5A). Glucose acted more strongly than sucrose, in contrast to WT. The amplitude of the accumulation response was reduced by 24% (20%) by glucose (sucrose) (Fig. 5D, left upper graph, col. 2,3) and the velocity was reduced by 34% (23%), respectively (Fig. 5D, left bottom graph, col. 2,3). The avoidance response, mediated solely by phototropin2, remained almost unchanged after a two-day incubation with these sugars in PHOT2 overexpressor (Fig. 5D, right graphs). In WT leaves the movements were clearly inhibited under the same conditions. This could suggest that the sugar inhibition of chloroplast movements was mainly due to a downregulation of PHOT2 expression, whereas this inhibition was compensated by the excess of phot2 expressed under the strong 35S promoter. To verify this possibility the steady-state transcript level of PHOT2 in the WT Columbia plants was tested by semi-quantitive PCR, with 18S RNA as a reference. The results are presented in Figure 4. Glucose markedly upregulated PHOT2 expression, whereas in sucrose-treated leaves it was at the same level as in the control ones.
Figure 5.
(A–C) Representative chloroplast responses to continuous blue light in leaves of Arabidopsis thaliana; (A) wild type, (B) PHOT2 overexpressor and (C) gin2-1 after 2 days of incubation with sucrose, glucose and mannose. Arrows indicate start of illumination with weak (↓1,6 µmol m−2 s−1) and strong (↓120 µmol m−2 s−1) blue light. Mean parameters of chloroplast responses in (D) PHOT2 overexpressor and (E) gin2-1. Each column represents the average of six to twelve measurements: 1, control leaves, two days on agar; 2, leaves adapted with 3% glucose for two days; 3, leaves adapted with 3% sucrose for two days. Error bars represent SD. Asterisks denote the significance of differences (p-value calculated with the unpaired t-test, *p = 0.01-0.05; **p = 0.01-0.001; ***p < 0.001).
Sugar regulation of senescence-associated genes in WT and PHOT2 overexpressor.
As was mentioned before, sugar inhibition was observed only in the leaves of mature plants. Senescence is one of the processes affected by sugars.29,30 To test the possibility that sugar perturbations of chloroplast responses in WT were simply caused by senescence, we compared the expression of two senescence-associated genes (SAGs): SAG12 and SAG13 in the leaves of WT and of PHOT2 overexpressor plants. SAG12 is often used as a marker of late senescence because it is expressed only in leaves with clear symptoms of senescence, e.g., yellowing. Its expression is insensitive to artificial treatments promoting the transcription of other SAGs. Thus, SAG12 is thought to be a specific indicator of natural senescence. The expression of SAG13 precedes visible senescence and increases with the progress of yellowing.31
In our experiments no mRNA of SAG12 was detectable in sugar-treated leaves (Fig. 6A). Attached leaves were darkened for 2 or 4 days in an additional control experiment by wrapping them in aluminum foil. The expression of SAG12 was markedly upregulated under these senescence-promoting conditions (Fig. 6B), in accordance with the results reported by Weaver and Amasino.32 Thus, the applied SAG12 assay worked correctly.
Figure 6.
Expression of SAG12 and SAG13 in A. thaliana WT Columbia and PHOT2 overexpressor leaves incubated with 3% sugars for 48h after 14 h of dark adaptation (A). Arabidopsis attached leaves darkened for 2 and 4 days were used as indicators of proper functioning of the SAG12 assay (B). The experiment was repeated twice with similar expression patterns being obtained.
The mRNA of SAG13, the second gene tested, was present in the control leaves, but its level was relatively low (Fig. 6A). The expression of SAG13 was strongly upregulated by glucose but not by sucrose. The latter sugar did not produce any changes in the amount of its mRNA as compared with the control. No differences were found either between the mRNA levels or expression patterns of SAG13 in WT and PHOT2 overexpressing plants (Fig. 6A).
Role of hexokinase in the sugar effect.
A dual function of Arabidopsis hexokinase1 in metabolizing and sensing hexose has been demonstrated recently.11 It is the only plant sugar sensor identified so far. To examine its role in the sugar effect we used hexokinase inhibitors and 3-O-methylglucose. The latter glucose analog has been commonly used to exclude the involvement of hexokinase in signal transduction as it does not activate a hexokinase-dependent pathway.33 In contrast to glucose and sucrose, 3-O-methylglucose hardly affected chloroplast responses in Lemna after two days (Fig. 2, col. 3). This points to a potential hexokinase involvement in the signal transduction pathway.
The application of mannoheptulose and glucosamine, two typical hexokinase inhibitors, led to completely divergent effects. Chloroplast responses to continuous blue light were intensified in Arabidopsis leaves treated with 0.02% mannoheptulose while they were almost eradicated in the presence of 0.02% glucosamine (Fig. 3, col. 14). The glucosamine effect was weaker in Lemna fronds, but the level of inhibition was still almost 60% for the accumulation response and 24% for the avoidance response (0.02% glucosamine, two days Fig. 2, col. 9). The results imply that these two inhibitors act in a different way, and point to some strong side effects of glucosamine.
To further elucidate the role of hexokinase we tested the hexokinase1 null mutant gin2-1. Although the mutant had a Landsberg erecta background whereas all other plants had a Columbia background, the results could be compared: no significant differences between chloroplast relocations have been found in Landsberg erecta and Columbia ecotypes under our test conditions (unpublished results).
Glucose and sucrose markedly reduced the chloroplast response to weak light in gin2-1 mutant (Fig. 5C and E). The amplitude and velocity of this response was lowered, similar to WT plants. The effects of these sugars on the avoidance response were different in gin2-1 mutant and in WT. The velocity of the avoidance response was lowered in the mutant (by 50% by glucose and 30% by sucrose, Fig. 5E bottom, right graph, col. 2, 3). The amplitude, however, remained unaffected (Fig. 5C and E upper left graph), in contrast to wild type, where it was reduced by about 40% (cf Fig. 5A and 4 col. 8,12). Thus, the dynamics of the response was impaired, but finally chloroplasts reached the same stationary position as in the sugar-untreated leaves.
Mannose was very strongly inhibitory in the case of gin2-1 as with WT: 1% mannose was sufficient to abolish both types of chloroplast relocations (Figs. 5C and A respectively).
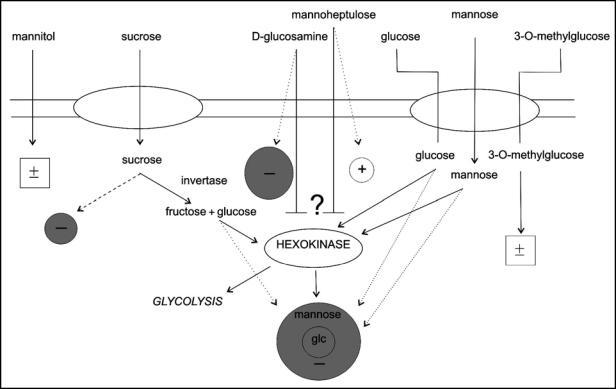
The results obtained in the present study are summarized in Figure 7. Dotted lines represent a possible direct impact of hexoses on chloroplast movements without activating a hexokinase-dependent pathway. Sucrose may act directly (dashed line), through a sucrose specific sensor, or after enzymatic conversion to glucose activating a hexose-specific signaling cascade. The digestion by invertase may take place outside or inside the cell. The lack of effect of 3-O-methylglucose on chloroplast relocations (marked with ±) indicates the participation of a hexokinase-mediated pathway in the glucose inhibition.
Figure 7.
The influence of sugars and sugar analogs on blue light-controlled chloroplast relocations—summary of the results. Grey circles denote inhibitory effects, an open circle represents the amplification of response. Sizes of circles reflect the magnitudes of the effects. The ± symbols indicate no influence. Dashed lines represent a potential direct effect of sucrose. Hypothetical hexokinase-mediated effects are marked with solid lines and hexokinase-independent effects with dotted lines.
Discussion
To our knowledge, this is the first report on the sugar inhibition of chloroplast movements. Sugars affected WT and mutant plants differently. Thus, their effects were not due to changes in the physical properties of cells (e.g., increased viscosity of the cytoplasm). Although sugar signaling in plants has been intensively investigated, no optimal experimental procedures have been established which facilitate unequivocal results. Sugars may act as an energy source or signaling molecules activating specific transduction pathways. To distinguish between these two roles, non-metabolizable sugar analogs are commonly used. However their uptake and metabolism are not well characterized for Arabidopsis,14 as reflected also by our results. Therefore, the use of mutants seems to be more conclusive. The different effects of sugars on chloroplast relocations in WT as compared with gin2-1 and PHOT2 overexpressor suggest the involvement of a sugar-specific transduction pathway rather than just a metabolic and/or stress effect. We discuss here several sugar signaling pathways which may be potentially involved. Further detailed investigations are necessary to elucidate the mechanism of sugar inhibition.
The sugar effects are not immediate, which points to changes in the expression of genes involved in chloroplast movements. Phototropins and the actomyosin system are required for these movements. Preliminary experiments have shown that the actin cytoskeleton is not affected by glucose/sucrose (unpublished data). The influence of sugars on PHOT2 expression is discussed below. The other components of the signal transduction pathway resulting in blue light-induced chloroplast movements have not yet been defined. Although some mutants have been isolated, no data are available on the role of the affected genes in the signaling cascade.
Influence of sugars on the functioning of Phototropin2.
In PHOT2 overexpressor the effect of sugars was clearly weaker than in WT, suggesting a compensation by an enhanced expression of phototropin2. This compensation was much stronger (almost complete) in the case of the avoidance response controlled only by phot2, in contrast to the accumulation response controlled by both phototropins. This provides evidence that some event(s) at or downstream of phototropin2 was (were) the target of sugar action.
In WT plants the expression of PHOT2 is organ specific,5 whereas it is high and unspecific in overexpressor plants. PHOT2 overproduction throughout plant life may influence some processes, as well as responses to external factors. The inhibition of chloroplast movements was not due to the suppression of PHOT2 transcription (and/or transcript stability) because neither glucose nor sucrose downregulated PHOT2 expression. However, sugars might cause destabilization or inactivation of the phot2 receptor. Such a destabilizing effect may be overriden by a higher amount of protein in overexpressor plants. Phototropins bind to the plasma membrane using ionic and/or covalent bonds7 and sugars might influence the membrane composition. A surplus of the PHOT2 protein in overexpressor plants may compensate for sugar-limited downstream components and/or membrane constituents involved in PHOT2 binding to the plasma membrane. It is certainly possible that a longer exposure to sugar might be necessary to impair chloroplast movements in PHOT2 overexpressor, but this could not be tested in experiments with detached leaves.
Sugar-specific anthocyanin accumulation was indistinguishable in WT and PHOT2 overexpressor plants. This indicates that the insensitivity of overexpressor chloroplasts to sugars was not due to their lower (or lack of) penetration into leaf cells. Sugar-responsive genes: CABs and UGP were regulated in WT by glucose and/or sucrose in the predicted way.25,26 The sugar response of MYB4 was contrary to data from the literature:28 in our experimental model its expression was promoted by glucose and not by sucrose. Thus, MYB4 mRNA turned out to be an inappropriate indicator for monitoring sucrose activity in detached leaves exposed to sugars. Dissimilarities in sucrose regulation might have been caused by very different growth conditions or the growth stage of plants used in the two experimental models. Sugars may regulate some cellular processes in different ways depending on their delivery—by the vascular system from roots, or simply by diffusion, as in our experiments. Jin's group used 12 day old Arabidopsis plants grown in vitro on an MS medium supplemented with 100 mM sucrose. Light conditions during adaptation to sucrose might also have been different but that aspect of experiments is not commented on in the cited paper. The same expression pattern of MYB4 and PHOT2 in the presence of glucose or sucrose may imply the involvement of MYB4 as the transcription factor in PHOT2 regulation, but this hypothesis needs further verification.
Hexokinase1 mediates reduction of the avoidance response amplitude.
Sugar signaling proceeds along various pathways in plant cells. Sucrose and other disaccharides activate signaling pathways different from those stimulated by hexoses.34 Therefore, the almost identical effects of glucose and sucrose on light-induced chloroplast relocations may be interpreted in terms of sucrose operating actually as a hexose after digestion by invertases.
As with yeast and mammals, hexokinase is a factor in one of the hexose-induced signaling pathways in plant cells.14 The hexokinase inhibitors used in this work had widely varying effects on chloroplast movements. Both accumulation and avoidance responses were intensified in Arabidopsis WT in the presence of mannoheptulose, whereas they were almost totally repressed in leaves treated with glucosamine under the same conditions. Glucosamine inhibition of movement, particularly of the accumulation response, was much weaker in Lemna, which might be connected with the lower membrane permeability of duckweed. Our results lead to the conclusion that these two hexokinase inhibitors function differently and can not replace each other.
Although glucosamine is commonly acknowledged as a hexokinase inhibitor, it can also influence other processes in plant cells. Glucosamine can act as a protein kinase inhibitor; it has been shown to decrease glucose-induced MAPK activity, independent of any hexokinase pathway.35 Additionally, glucosamine impairs the xanthophyll cycle in Lemna along with other aminosugars,36 and completely inhibits primary root elongation in Arabidopsis seedlings at a concentration of 0.3%.37 Thus glucosamine definitely cannot be regarded as a specific hexokinase inhibitor.
Mannoheptulose, often used to investigate the role of hexokinase in sugar sensing, occurs naturally in many plant species and its level may be very high as, for example, in avocado. Mannoheptulose is found in the avocado phloem, along with sedoheptulose, and a decrease of its level is a prerequisite for the fruit ripening.38 The promotion of chloroplast responses by mannoheptulose without exogenously delivered sugars, can be due to its inhibition of the hexokinase-dependent signaling pathway activated by endogenous hexoses. Also some other physiological functions of mannoheptulose in addition to blocking hexokinase activity cannot be excluded in Arabidopsis.
Exposure to 3-O-methylglucose caused only a slight diminution of chloroplast responses to continuous blue light in Lemna. Although in maize this sugar analog is phosphorylated by hexokinase, it is not a respiratory and growth substrate and does not activate a hexokinase-dependent pathway.33 The different effects of sucrose and glucose, as compared with 3-O-methylglucose, support the involvement of hexokinase in the sugar inhibition of chloroplast relocations.
As mentioned above, glucosamine and mannoheptulose were unsuitable as inhibitors of sugar-activated hexokinase-mediated pathways under our experimental conditions. More conclusive evidence was obtained in experiments with gin2-1, an Arabidopsis hexokinase1 null mutant. Sucrose and glucose that impaired both chloroplast responses in WT plants left the amplitudes of the avoidance response unaffected in the mutant. It has been shown previously that distinct signaling pathways exist for accumulation and avoidance responses, and separate mechanisms control their amplitudes and velocities.39,40 Hexokinase1 appears to be involved in a sugar-induced signaling cascade leading to a reduction in avoidance response amplitude.
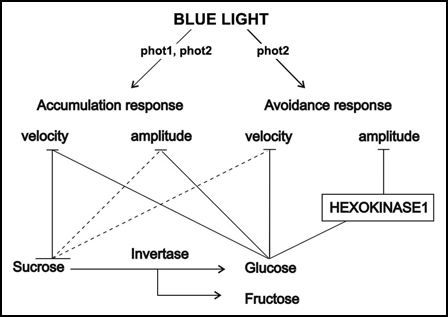
Figure 8 shows a hypothetical site of hexokinase1 action in the sugar inhibition of chloroplast relocations. According to the result obtained with the gin2-1 mutant, sucrose and glucose most probably influence the accumulation response and the velocity of the avoidance response via a hexokinase1-independent pathway. There are six hexokinase-like genes in Arabidopsis. In experiments investigating mutants with changed sensitivity to sugars, only hexokinase1 has been found to act as a glucose sensor so far.11 Nevertheless, it is very probable that some other hexokinases, or glycolysis-associated signals are functional in inhibiting chloroplast relocations.
Figure 8.
Hypothetical involvement of hexokinase1 in the sugar inhibition of chloroplast movements. Dashed lines indicate potential direct effects of sucrose.
The downstream elements of the hexokinase-dependent signaling pathway in plants are unclear. In our experiments sugar effects were observed after prolonged exposure, indicating a possible modulation of gene expression. A portion of hexokinase1 has been found associated with the nucleus, suggesting that it might be directly involved in the control of transcription.41
Another hexose tested, mannose, is often used to exclude metabolic effects in sugar-induced responses. Its phosphorylation by hexokinase leads to the production of mannose-6-phosphate that does not enter glycolysis. Consequently, Pi deficiency is sometimes observed as a side effect of mannose feeding. Mannose affected chloroplast relocations in Lemna and WT Arabidopsis much more strongly than glucose and sucrose. Moreover, it canceled both types of chloroplast responses in the gin2-1 mutant, while glucose and sucrose affected only the velocity of the avoidance response. Therefore, the mechanism of mannose inhibition must be different from that produced by these two sugars. D-mannose can lead to programmed cell death in plant cells by activating a DNA endonuclease and releasing cytochrome c from mitochondria.42 Thus we cannot rule out the possibility that the strong effect of mannose was indirect and due to perturbed cell homeostasis.
Age-dependence.
Our results lead to the conclusion that the components responsible for the sugar effect may be inactive/absent in younger plants. Sugars impaired blue light-induced chloroplast relocations only in Arabidopsis plants older than five weeks, right before bolting, indicating that they are involved in movement control only at a specific life phase. Various facts suggest that different sugar-induced signaling pathways are active at different developmental stages. The autophosphorylation activity of several potential protein kinases in tobacco depends on plant age and is higher in the top, younger leaves.23 Mutants gin2 and abi4, which are insensitive to glucose at later developmental stages, show glucose inhibition of germination similar to WT plants.43 Shift experiments have demonstrated that sucrose delays floral transition in wild type Arabidopsis only if delivered at later stages of the vegetative phase.44 Age-dependent components of a signaling cascade, i.e., components only active at a later growth phase, may be necessary for the inhibition by sugars of chloroplast movements.
Sugars have been shown to initiate and accelerate senescence.29,30 Molecular studies of this process are mainly focused on the expression of SAGs. The expression of SAG12 has been shown to be extremely intensified in an age-dependent manner during the growth of Arabidopsis on a glucose-enriched medium with a low nitrogen supply.45 In our case, sugars did not upregulate SAG12 expression. No mRNA of this gene was detectable in WT or in PHOT2 overexpressor leaves. Neither was it in the control, nor those incubated with glucose and/or sucrose, which is compatible with the lack of senescence symptoms (yellowing) in these leaves.
In contrast to SAG12, the expression of SAG13 was detectable in detached Arabidopsis leaves incubated for two days on agar. Glucose strongly enhanced the accumulation of SAG13 mRNA, whereas sucrose did not influence the expression of this gene. A marked upregulation of SAG13 expression by glucose and sucrose has been reported in detached Arabidopsis leaves under continuous illumination.46 Probably light-treatment is crucial for sucrose-induced upregulation.
The same mode of SAG13 upregulation was observed in WT and PHOT2 overexpressing plants. Moreover, only glucose promoted expression of SAG13, although the inhibitory impact of glucose and sucrose on chloroplast movements was identical. Thus, senescence was not the reason for chloroplast movement inhibition by sugars.
The biological significance of the sugar-induced inhibition of chloroplast responses remains unclear. An elevated sugar level may be sensed as a stress condition connected with a disturbed source/sink balance. An accumulation of sugars, preceded by the activation of invertases, has been observed during pathogen attack. Moreover, sugars may induce the transcription of pathogen-related genes. This transcription is age-dependent and occurs only in mature leaves.47 Many aspects of the interactions between pathogens and plants have been investigated, but chloroplast movements have not been studied in this context. Movement responses are energy-consuming, and probably plants preferentially direct energy into more vital processes under stress conditions.
Acknowledgements
This work was supported by grant no. PB320/PO4 2003/25 of the State Committee for Scientific Research (KBN, Poland). We are sincerely grateful to Dr. Barbara Karpinska for helping us with planning and undertaking the study of RNA expression and to Dr. J. Jarillo for providing the seeds of PHOT2 overexpressor.
Abbreviations
- CABs
chlorophyll a/b binding proteins
- MAPK
mitogen activated protein kinase
- PHOT
phototropin
- SAG
senescence associated gene
- WT
wild type
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4392
References
- 1.Zurzycki J. Blue light-induced intracellular movements. In: Senger H, editor. The Blue Light Syndrome. Berlin, Heidelberg, New York: Springer Verlag; 1980. pp. 50–68. [Google Scholar]
- 2.Kasahara M, Kagawa T, Oikawa K, Suetsugu N, Miyao M, Wada M. Chloroplast avoidance movement reduces photodamage in plants. Nature. 2002;420:829–832. doi: 10.1038/nature01213. [DOI] [PubMed] [Google Scholar]
- 3.Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K. Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature. 2001;414:656–659. doi: 10.1038/414656a. [DOI] [PubMed] [Google Scholar]
- 4.Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K. Arabidopsis nph1 and npl1: Blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA. 2001;98:6969–6974. doi: 10.1073/pnas.101137598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarillo JA, Gabrys H, Capel J, Alonso JM, Ecker JR, Cashmore AR. Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature. 2001;410:952–954. doi: 10.1038/35073622. [DOI] [PubMed] [Google Scholar]
- 6.Sakamoto K, Briggs WR. Cellular and subcellular localization of phototropin 1. Plant Cell. 2002;14:1723–1735. doi: 10.1105/tpc.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knieb E, Salomon M, Rüdiger W. Tissue-specific and subcellular localization of phototropin determined by immuno-blotting. Planta. 2004;218:843–851. doi: 10.1007/s00425-003-1164-7. [DOI] [PubMed] [Google Scholar]
- 8.Koch KE. Carbohydrate modulated gene expression in plants. Annu Rev Plant Phys Plant Mol Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- 9.Rolland F, Moore B, Sheen J. Sugar sensing and signaling in plants. Plant Cell. 2002;14:185–205. doi: 10.1105/tpc.010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riou-Khamlichi C, Menges M, Healy JMS, Murray JAH. Sugar control of the plant cell cycle: Differential regulation of Arabidopsis D-type cyclin gene expression. Mol Cell Biol. 2000;20:4513–4521. doi: 10.1128/mcb.20.13.4513-4521.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao W, Sheen J, Jang JCH. The role of hexokinase in plant sugar signal transduction and growth and development. Plant Mol Biol. 2000;44:451–461. doi: 10.1023/a:1026501430422. [DOI] [PubMed] [Google Scholar]
- 12.Lalonde S, Boles E, Hellmann H, Barker L, Patrick JW, Frommer WB, Ward JM. The dual function of sugar carriers: Transport and sugar sensing. Plant Cell. 1999;11:707–726. doi: 10.1105/tpc.11.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinha AK, Hoffman MG, Römer U, Köckenberger W, Elling L. Metabolizable and non-metabolizable sugars activate different signal transduction pathways in tomato. Plant Physiol. 2002;128:1480–1489. doi: 10.1104/pp.010771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson SI. Plant sugar-response pathways: Part of a complex regulatory web. Plant Physiol. 2000;124:1532–1539. doi: 10.1104/pp.124.4.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genoud T, Métraux JP. Crosstalk in plant cell signalling: Structure and function of the genetic network. Trends Plant Sci. 1999;12:503–507. doi: 10.1016/s1360-1385(99)01498-3. [DOI] [PubMed] [Google Scholar]
- 16.Ciereszko I, Kleczkowski LA. Effects of phosphate deficiency and sugars on expression of rab 18 in Arabidopsis: Hexokinase-dependent and okadaic acid-sensitive transduction of the sugar signal. Biochim Biophys Acta. 2002;1579:43–49. doi: 10.1016/s0167-4781(02)00502-x. [DOI] [PubMed] [Google Scholar]
- 17.Coruzzi GM, Zhou L. Carbon and nitrogen sensing and signalling in plants: Emerging “matrix effects”. Curr Opi Plant Biol. 2001;4:247–253. doi: 10.1016/s1369-5266(00)00168-0. [DOI] [PubMed] [Google Scholar]
- 18.Thum KE, Shasha DE, Lejay LV, Coruzzi GM. Light- and carbon-signaling pathways: Modeling circuits of interactions. Plant Physiol. 2003;132:440–452. doi: 10.1104/pp.103.022780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lejay L, Gansel X, Cerezo M, Tillard P, Müller C, Krapp A, von Wirén N, Daniel-Vedele F, Gojon A. Regulation of root ion transporters by photosynthesis: Functional importance and relation with hexokinase. Plant Cell. 2003;15:2218–2232. doi: 10.1105/tpc.013516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Short TW. Overexpression of Arabidopsis phytochrome B inhibits phytochrome A function in the presence of sucrose. Plant Physiol. 1999;119:1497–1505. doi: 10.1104/pp.119.4.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appenroth KJ, Teller S, Horn M. Photophysiology of turion and germination in Spirodela polyrhiza. Biol Plantarum. 1996;38:95–106. [Google Scholar]
- 22.Walczak T, Gabrys H. New type of photometer for measurements of transmission changes corresponding to chloroplast movements in leaves. Photosynthetica. 1980;14:65–72. [Google Scholar]
- 23.Ohto M, Nakamura K. Sugar-induced increase of calcium-dependent protein kinases associated with the plasma membrane in leaf tissues of tobacco. Plant Physiol. 1995;109:973–981. doi: 10.1104/pp.109.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsukaya H, Ohshima T, Naito S, Chino M, Komeda Y. Sugar-dependent expression of the CHS-A gene for chalcone synthase from petunia in transgenic Arabidopsis. Plant Physiol. 1991;97:1414–1421. doi: 10.1104/pp.97.4.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheen J. Metabolic repression of transcription in higher plants. Plant Cell. 1990;2:1027–1038. doi: 10.1105/tpc.2.10.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciereszko I, Johansson H, Kleczkowski LA. Sucrose and light regulation of a cold-inducible UDP-glucose pyrophosphorylase gene via a hexokinase-independent and abscisic acid-insensitive pathway in Arabidopsis. Biochem J. 2001;354:67–72. doi: 10.1042/0264-6021:3540067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kranz HD, Denekamp M, Greco R, Jin H, Leyva A, Meissner RC, Petroni K, Urzainqui A, Bevan M, Martin C, Smeekens S, Tonelli CH, Paz-Ares J, Weisshaar B. Technical advance towards functional characterisation of the members of the R2R3-MYB gene family from Arabidopsis thaliana. Plant J. 1998;16:263–276. doi: 10.1046/j.1365-313x.1998.00278.x. [DOI] [PubMed] [Google Scholar]
- 28.Jin H, Cominelli E, Bailey P, Parr A, Mehrtens F, Jones J, Tonelli CH, Weisshaar B, Martin C. Transcriptional repression by AtMYB 4 controls production of UV-protecting sunscreens in Arabidopsis. EMBO J. 2000;19:6150–6161. doi: 10.1093/emboj/19.22.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wingler A, von Schaewen A, Leegood RC, Lea PJ, Quick WP. Regulation of leaf senescence by cytokinin, sugars, and light. Plant Physiol. 1998;116:329–335. [Google Scholar]
- 30.Wingler A, Purdy S, MacLean JA, Pourtau N. The role of sugars in integrating environmental signals during the regulation of leaf senescence. J Exp Bot. 2006;57:391–399. doi: 10.1093/jxb/eri279. [DOI] [PubMed] [Google Scholar]
- 31.Weaver LM, Gan S, Quirino B, Amasino RM. A comparison of the expression patterns of several senescence associated genes in response to stress and hormone treatment. Plant Mol Biol. 1998;37:455–469. doi: 10.1023/a:1005934428906. [DOI] [PubMed] [Google Scholar]
- 32.Weaver LM, Amasino RM. Senescence is induced in individually darkened arabidopsis leaves, but inhibited in whole darkened plants. Plant Physiol. 2001;127:876–886. [PMC free article] [PubMed] [Google Scholar]
- 33.Cortès S, Gromova M, Evrard A, Roby C, Heyraud A, Rolin DB, Raymond P, Brouquisse RM. In plants, 3-O-methylglucose is phosphorylated by hexokinase but not perceived as a sugar. Plant Physiol. 2003;131:824–837. doi: 10.1104/pp.010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rook F, Gerrits N, Kortstee A, van Kampen M, Borrias M, Weisbeek P, Smeekens S. Sucrose-specific signalling represses translation of the Arabidopsis ATB2 bZIP transcription factor gene. Plant J. 1998;15:253–263. doi: 10.1046/j.1365-313x.1998.00205.x. [DOI] [PubMed] [Google Scholar]
- 35.Hofmann M, Roitsch T. The hexokinase inhibitor glucosamine displays a dual effect on protein kinase activity in vitro. J Plant Physiol. 2000;157:13–16. [Google Scholar]
- 36.Latowski D, Banas AK, Strzalka K, Gabrys H. Amino sugars—New inhibitors of zeaxanthin epoxidase, a violaxanthin cycle enzyme. J Plant Physiol. 2006 doi: 10.1016/j.jplph.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Baskin TI, Remillong EL, Wilson JE. The impact of mannose and other carbon sources on the elongation and diameter of the primary root of Arabidopsis thaliana. Aust J Plant Physiol. 2001;28:481–488. [Google Scholar]
- 38.Liu X, Sievert J, Arpaia ML, Madore MA. Postulated physiological roles of the seven-carbon sugars, mannoheptulose, and perseitol in Avocado. J Am Soc Horti Sci. 2002;127:108–114. [Google Scholar]
- 39.Tlalka M, Gabrys H. Influence of calcium on blue-light-induced chloroplast movement in Lemna trisulca L. Planta. 1993;189:491–498. [Google Scholar]
- 40.Grabalska M, Malec P. Blue light-induced chloroplast reorientations in Lemna trisulca L. (Duckweed) are controlled by two separable cellular mechanisms as suggested by different sensitivity to wortmannin. Photochem Photobiol. 2004;79:343–348. doi: 10.1562/le-03-16.1. [DOI] [PubMed] [Google Scholar]
- 41.Yanagisawa S, Yoo SD, Sheen J. Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature. 2003;425:521–525. doi: 10.1038/nature01984. [DOI] [PubMed] [Google Scholar]
- 42.Stein JC, Hansen G. Mannose induces an endonuclease responsible for DNA laddering in plant cells. Plant Physiol. 1999;121:71–79. doi: 10.1104/pp.121.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Price J, Li T, Kang SG, Na JK, Jang J. Mechanisms of glucose signaling during germination of Arabidopsis. Plant Physiol. 2003;132:1424–1438. doi: 10.1104/pp.103.020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohto M, Onai K, Furukawa Y, Aoki E, Araki T, Nakamura K. Effects of sugar on vegetative development and floral transition in Arabidopsis. Plant Physiol. 2001;127:252–261. doi: 10.1104/pp.127.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pourtau N, Jennings R, Pelzer E, Pallas J, Wingler A. Effect of sugar-induced senescence on gene expression and implications for the regulation of senescence in Arabidopsis. Planta. 2006;224:556–568. doi: 10.1007/s00425-006-0243-y. [DOI] [PubMed] [Google Scholar]
- 46.Noh YS, Amasino RM. Identification of a promoter region responsible for the senescence-specific expression of SAG12. Plant Mol Biol. 1999;41:181–194. doi: 10.1023/a:1006342412688. [DOI] [PubMed] [Google Scholar]
- 47.Herbers K, Sonnewald U. Altered gene expression brought about by inter-and intracellularly formed hexoses and its possible implications for plant-pathogen interactions. J Plant Res. 1998;111:323–328. [Google Scholar]