Abstract
Exposure to the allelopathic monoterpenes camphor (100 mg/10 L) and menthol (50 mg/10 L) for 24 h enhanced transpiration of Arabidopsis thaliana fully developed rosette leaves similar to de-waxing. As ascertained by ESEM analyses the leaf surfaces were spotted with platelet like structures which seem to be partly mixed with the lipophilic epicuticular layers. The structures are supposed to contain the condensed monoterpenes, which could be identified by GC. Long term exposure (more than 48 h) to 100 mg/50 mg killed the plants by desiccation, a 24 h exposure caused necrotic spots that became visible one to two days after the treatment. Examinations of the stomatal apertures indicated that monoterpenes induced stomatal opening followed by extreme swelling and a final break down of the protoplasts. Exposure of Arabidopsis thaliana to volatiles of Mentha piperita, Lavandula latifolia and Artemisia camphorata resulted in a dramatic increase of the stomata aperture but swelling of the protoplasts was less exhibited.
In contrast to de-waxing, expression of the fatty acid condensing enzyme encoding CER6 gene and de novo synthesis of CER6 protein was not induced after 24 h of exposure to the monoterpenes.
The aim of the study was to demonstrate that the lipophilic layers of the leaf surface and the stomata are primary targets of monoterpene allelopathic attack. Enhanced transpiration results from a combination of affected lipophilic wax layers and a disturbed stomata function.
Key Words: Allelopathy, terpenes, cuticular waxes, CER6, stomata, Arabidopsis thaliana, Artemisia camphorata, Lavandula latifolia, Mentha piperita
Introduction
Many aromatic plants emit volatile monoterpenes with allelopathic potential. The most prominent example of an ecological phenomenon caused by terpenes are bare zones around the shrubs of Salvia leucophylla and Artemisia californica, both dominant species in the coastal strip vegetation in Southern California (High Chaparral). Within the bare zones no species of the Poaceae from the surrounding grassland is able to survive. The phenomenon was extensively studied already in the sixties of the last century by Muller and coworkers.1–3 They identified a number of terpenoids in the air above the salvia shrubs including cineols and camphor. It was assumed that these compounds are most likely responsible for the observed growth inhibition around the shrubs. A clear demonstration of terpenes as allelochemicals that can have toxic effects on target plants, dependent on the concentration, was given by the studies of Richardson and Williamson, Vokou et al. and Barney et al.4–6
A higher solubility of monoterpenes in water as hitherto assumed was found by Weidenhamer et al. (1993).7 Thus, certain phytotoxic effects of monoterpenes may be developed by water-soluble quantities of the compounds, which should be of importance for effects within an aqueous environment such as the cytoplasm of plant cells. Nevertheless, the molecular mode of action of most monoterpenes is not well investigated yet although monoterpenes are wide-spread secondary products in plant kingdom and allelopathic interaction due to the compounds might be common. Detailed studies are only published for cineols and camphor. These compounds are strongly growth inhibitory, e.g., 1,8-cineol inhibits mitosis, leads to growth abnormities, inhibits respiration of isolated mitochondria and aspartate synthase in concentrations above 1 µM.8–11 Camphor is supposed to act in a similar manner. Deterioration of membrane integrity is another known effect.12 Furthermore, the compounds can have different cellular targets depending on their existence as volatile compounds in the air or as components of an aqueous solution. As volatiles, the lipophilic character of the compounds should be of a greater importance. If this is true, the first target of a volatile terpene can be the plant surface as aerial plant surfaces are covered by apolar substances in the cuticle. Stomata can be another target.
The surface components, cuticle and cuticular waxes, are highly lipophilic layers which is in accordance with their major function: to protect the plant against uncontrolled water loss and transpiration.13 In addition, cuticular waxes are also protective against UV-irradiation, frost damage and other negative influences such as pathogenic attacks.14,15
In Arabidopsis several very long chain fatty acids (VLCFAs) condensing enzymes have been identified. Three of them, CER6, FDH and KCS1, are involved in the synthesis of VLCFA for wax production.16–19 One of these enzymes, CER6 (CUT1) has been found as one of the factors controlling wax accumulation in A. thaliana.18,20 It is therefore considered as the key enzyme for wax biosynthesis that has no significant functional overlap with KCS1 and FDH activities. CER6 is expressed in different amounts throughout the shoot, during all stages of stem and leaf development from 1-day-old seedling to 14-day-old ones.21 Moreover, transcription of CER6 is stimulated by light and osmotic stress. Both is in agreement with consensus sequences for light and ABRE (ABA-responsive cis-acting elements) present in the promotor of CER6. Therefore, Hooker et al hypothesized that wax accumulation in Arabidopsis is controlled by the level of CER6 transcription.21
Predominant constituents of cuticular waxes are primary and secondary alcohols, aldehydes, ketones, alkanes and esters, which are derived from VLCFAs. Wax biosynthesis, which appears exclusively in the epidermal cells, starts with the elongation of C18:0 fatty acids to wax precursors up to 34 carbons in length. Details of the fatty acid elongation and further steps of the complex synthesis of cuticular wax constituents have been reviewed by several authors.22,23 At least in Arabidopsis, the wax export as a final step out of the epidermal cells, requires a plasma membrane localized ABC transporter encoded by the CER5 gene.24,25
The chemical nature of waxes suggests the hypothesis that monoterpenes interact with these plant surface structures as a primary site from which further allelopathic attacks are initiated. In this study, the influence of the monoterpenes camphor and menthol on CER6 gene transcription in Arabidopsis rosette leaves in comparison with untreated plants and those where cuticular waxes have been removed, was investigated. A possible deposition of the monoterpenes on leaf surfaces and resulting consequences were studied as well as effects on transpiration rates. As there is hardly any information available about effects on stomata, stomatal reactions were investigated by monitoring alterations in the size of stomatal pores after exposure to volatiles of Artemisia camphorata, Lavandula latifolia and Mentha piperita.
The aim of this study is to demonstrate that lipophilic layers of the plant surface and guard cells are among the first targets affected by volatiles and the monoterpenes camphor and menthol. Beside the subsequent destruction of cells, dessiccation is supposed to be one major reason responsible for deleterious effects of monoterpenes on plants.
Material and Methods
Plant Material.
Seeds of Arabidopsis thaliana (ecotype COL-0) were germinated and grown in garden compost for three weeks in a greenhouse. Single plants were then planted in pots and grown for another week before using for experiments.
Removal of cuticular wax layers.
Cuticular waxes were removed as described by Neinhuis et al.26
Monoterpene treatment.
For treatments with camphor and menthol, respectively, each four plants, were transferred into a 10 L exsiccator for 12 h (48h). Petri dishes with two combinations of solid camphor and menthol (50 mg camphor/25 mg menthol; 100 mg camphor/50 mg menthol) were placed between the pots containing single Arabidopsis plants. Under the used conditions, evaporation of the monoterpene crystals was 39–42 mg for camphor and about 10–12 mg for menthol within 24 h. When exposure was continued up to 96 h, further charges of monoterpenes was placed into the chamber. Circulation of air was guaranteed by small ventilators inside of the exsiccators and exchange with the environment outside by two small openings. Controls were run under identical conditions but without monoterpene addition.
Identification of camphor and menthol on leaf surfaces.
300 mg young and old rosette leaves in mixture were harvested from monoterpene-treated and control plants and immersed in 1 ml dichlormethane. The samples were analyzed by GC (Hewlett Packard Typ 5890 with a 7673A sampler and a flame ionisation detector (FID). Components were separated on a 25 m BPX5 capillary column with a 0.25 mm inner diameter and 0.25 µm film thickness. The column was operated with N2 (primary pressure 0,1 MPa) as carrier gas. Samples (1 µl) were injected by split injection (split flow: 20 ml/min). The oven temperature was increased from 60 to 150°C at 3°C/min. Data were monitored with the EZ-chrom software. Monoterpenes were identified by comparison of retention times and coinjections of standard compounds.
Microscopical studies.
Rosette leaf surfaces were studied by environmental scanning electron microscopy (ESEM) after removal of wax layers and after exposure to the monoterpenes in comparison to the controls (XL 30 ESEM TMP, FEI Philips, Germany) using a gaseous secondary electron detector (GSED). The temperature was set to 3.7°C by dint of a Peltier cooling element in order to prevent artefacts and damages of the surface from the fresh and unprepared sample, caused by the vacuum or electron beam. The leaf samples were mounted and the edges sealed on the mount-assay by conductive carbon paint. Pictures were taken in the range of 17.0–23.5 kV acceleration voltage with spots between 3.8 and 4.1, the working distances were 7.9–10.8 mm and the pressure 0.8–6.8 hPa.
Transpiration measurements.
Transpiration measurements were performed after 24 h of monoterpene exposure with a steady-stateporometer (LI-6400, LICOR) using the Arabidopsis chamber (0.78 cm2). Calculation of the transpiration based on the formula E=F(Ws-Wr)/100S(1000.Ws) [E = transpiration rate (mol m −2 s−1), F = flow rate (µmol s−1, Ws = water fraction of the air influenced by the leaf (mmol H2O / mol air), Wr = water fraction of the reference (mmol / mol air), S = leaf area (cm2)]. For each experiment 12 independent measurements were taken at constant conditions (t = 26°C, rh = 40%, CO2 = 400 ppm). For statistical treatment of the data SPSS 12.0 software was used (Oneway Anova, Tukey-HSD, p < 0.001).
Exposure to volatiles of aromatic plants.
Arabidopsis thaliana plants were exposed to volatiles of Artemisia camphorata, Lavandula latifolia or Mentha piperita. Single plants (15 cm high) of the different species were placed into exsiccators together with one plant of Arabidopsis thaliana. Controls were set up without exposure to aromatic plants. Circulation and exchange of air was as described for monoterpene treatment. Artemisia camphorata and Lavandula latifolia were purchased from Rühlemann (Horstedt, Germany), Mentha piperita was grown in the Botanical Garden, University of Bonn. Rosette leaves of A. thaliana were harvested after 24, 48, 72 and 96 h, arranged upside down and covered with tape. The upper parts of the leaves were stripped and residues of the mesophyll carefully removed. The tapes with the lower epidermis were fixed at a slide and immediately used for microscopical measurements of stomatal apertures (Olympus BH2 with photographic equipment PM10 AD). A 10x okular SWHK was used for calibration. Data of the 24 h exposures were statistically treated with the SPSS 12.0 software (Oneway Anova, Tukey-HSD, p < 0.001).
Real time PCR.
mRNA was prepared from Arabidopsis rosette leaves of apparently identical physiological stage by sampling six leaves from different plants which were either untreated (control) or de-waxed (immediately after first de-waxing or 1.5 h after first de-waxing, twice de-waxed and used immediately or after 1, 2 and 3 h of recreation). For mRNA isolation from rosette leaves the Qiagen Oligotex direct mRNA Microkit was used. Terpene treatments were performed as described and mRNA prepared from untreated leaves (control), from leaves treated with 50 mg camphor/25 mg menthol directly after 24 h exposure and after 1.5 h of recreation and from leaves treated with 100 mg camphor/50 mg menthol directly after 24 h exposure and after 1.5 h of recreation. First strand cDNA was synthesized with the Sensiscript Qiagen Kit according to the manufacturerer's instructions using 50 ng of poly (A)+ RNA in a final volume of 20 µl reaction.
The presence of CER6 transcripts in the samples was checked by amplifying synthesised cDNA. A positive control was carried out by amplifying two standard house-keeping genes, actin act2 and elongation factor1. The total reaction volume for PCR was 20 µl and contained 1 µl cDNA, 1 U of Taq DNA polymerase (Fermentas), 2 µl of 10 × Taq buffer, 50 nmol MgCl2, 14 pmol of each primer (eF1-forward: 5′-AGG TCC ACC AAC CTT GAC TG-3′; eF1-reverse: 5′-GAG ACT CGT GGT GCA TCT CA-3′; act2-forward: 5′-TGC CAA TCT ACG AGG GTT TC-3′; act2-reverse: 5′-TTC TCG ATG GAA GAG CTG GT-3′; CER6-forward: 5′-ATC GAC GAG CTC CAA AGA A-3′; CER6-reverse: 5′-TTA CAT TTC CAC ACG GCA GA-3′) and 4 nmol of dNTP mixture. The PCR mixture was amplified using the iCycler (BioRad). A hot start incubation of 95°C was followed by 35 cycles of 95°C for 30 sec, 57.9°C (eF1), 55.2°C (act2), 53.5°C (CER6) for 60 sec and 72°C for 60 sec. After amplification, PCR products were visualized by standard agarose gel electrophoresis.
Real time PCR was conducted in an ABI Prism 7000 SDS instrument (Applied Biosystems) and SYBR green was used as a DNA specific fluorescent dye.
For the quantitative determination of CER6 transcripts by real time PCR, the following primers were designed (Primer Express Software v 2.0, Applied Biosystems): sense:
5′-GTG TCC CCT TCG CAA CTT TC-3′; antisense: 5′-TTC TCA TTT GGA ACT CGA CGC 3′. Primer optimization was performed prior to quantification. Standard curves were generated for the target gene using serial dilutions of plasmid (CER6) DNA (101–108 molecules) Prior to real time PCR the primers were tested by PCR. The products were checked for homogeneity and absence of artefacts by agarose gel electrophoresis and sequencing of the products.
Polymerase chain reactions were performed in 25 µl reaction volume containing 12.5 µl of 2X SYBR green universal master mix (QuantiTect™ SYBRR Green PCR KIT, Quiagen), optimal levels of forward and reverse primers and 2 µl cDNA. Each PCR run was performed in duplicate. A universal thermal cycling parameter (2 min at 50°C, 10 min at 95°C, 40 cycles of 15 sec at 95°C and 60 sec at 60°C) was used for the quantification.27 At the end of the last cycle, a dissociation curve was generated by starting the fluorescence acquisition at 60°C and taking measurements every 7 sec until the temperature reached 95°C. Final quantification analysis was performed using the standard curve method (ABI Prism 7700 SDS). Real time PCR experiments were repeated four times by two different experimentors with independently prepared samples. As normalizing data with internal standards such as commonly used β-actin mRNA, GAPDH mRNA or rRNA have been described as inappropriate, copy numbers were recorded as copy numbers per 50 ng cDNA.28,29 In addition, the presence CER6 in leaf protein extracts was checked immunologically.
Cloning of a CER6 fragment.
Specific primers were designed to amplify a 562 bp fragment of CER6. Primer sequences were as follows: sense: 5′ CCT CAG GCA CCG ATG CCA 3′; antisense: 5′ AAG GAT GTC GAC GTC TTA AGG 3′. PCR conditions used for CER6 fragment amplification were:
94°C for 5 min, 30 cycles of denaturation at 94°C for 1 min, annealing at 59.9°C for 45 sec, and extension at 72°C for 1 min, followed by a final extension at 72°C for 10 min in a Mastercycler® Gradient (Eppendorf).
The PCR product was separated by agarose gel electrophoresis, extracted (QIAquick Gel Extraction Kit) and cloned into Vector pQE-30VA. The construct was transformed into JM109 E.coli and M15 E.coli. Plasmids were isolated from the E.coli cultures (KIT) and checked by sequencing.
Expression of the CER 6 protein fragment.
Expression was induced by the addition of 0.4 mM IPTG to the growing M15 E.coli culture. The polypeptide was extracted in presence of 6 M urea and purified by His-tag binding resin columns (Qiagen Ni-NTA purification system). The eluted polypeptide was dialyzed against phosphate buffered saline (PBS) and concentrated by ultrafiltration. The obtained polypeptide was used to generate polyclonal antibodies in rabbits (Charles River laboratories). Sera with the highest antibody titer were precipitated with ammoniumsulfate, dialyzed against PBS and used for immuno-reactions without further purification.
Western blot analysis.
Fifty-100 mg of plant material was homogenized with liquid nitrogen. Proteins were extracted with SDS-sample buffer (4 µl / mg FW) in presence of 1 µl/mg FW protease inhibitor cocktail (Sigma). The mixture was further homogenized by mortaring with quartz sand. After boiling for 10 min, the homogenate was centrifuged for 15 min at 20°C and 10.000 g. The supernatant was used for SDS-PAGE (16.5%). Proteins, separated by SDS-PAGE, were transferred to nitrocellulose membrane (BioRad). For immunostaining 10 µl of CER6 fragment antiserum was used. Immunoreactive bands were visualized by a second incubation with alkaline phosphatase coupled anti-rabbit-IgGs from Sigma (1:1000).30 Purified recombinant CER6 fragment was used as a control.
Results
CER6 induction.
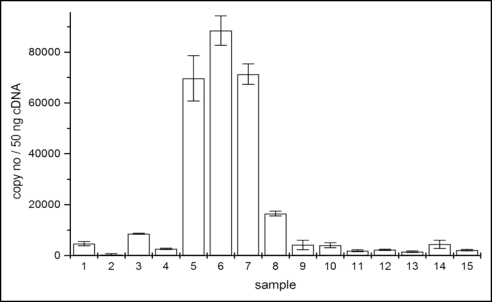
Examination of the cDNA by PCR points to differences in the CER6 gene expression depending on the time elapsed after de-waxing. Expression of elongation factor-1α and actin seem to increase slightly over time (Fig. 1). For CER6 expression, a more detailed information was obtained by real time PCR. In fully developed leaves, the level of CER6 transcripts was low, a higher expression was determined in young rosette leaves. Expression of CER6 in leaves of all stages of development with variations in the levels have also been described by Hooker et al.21
Figure 1.
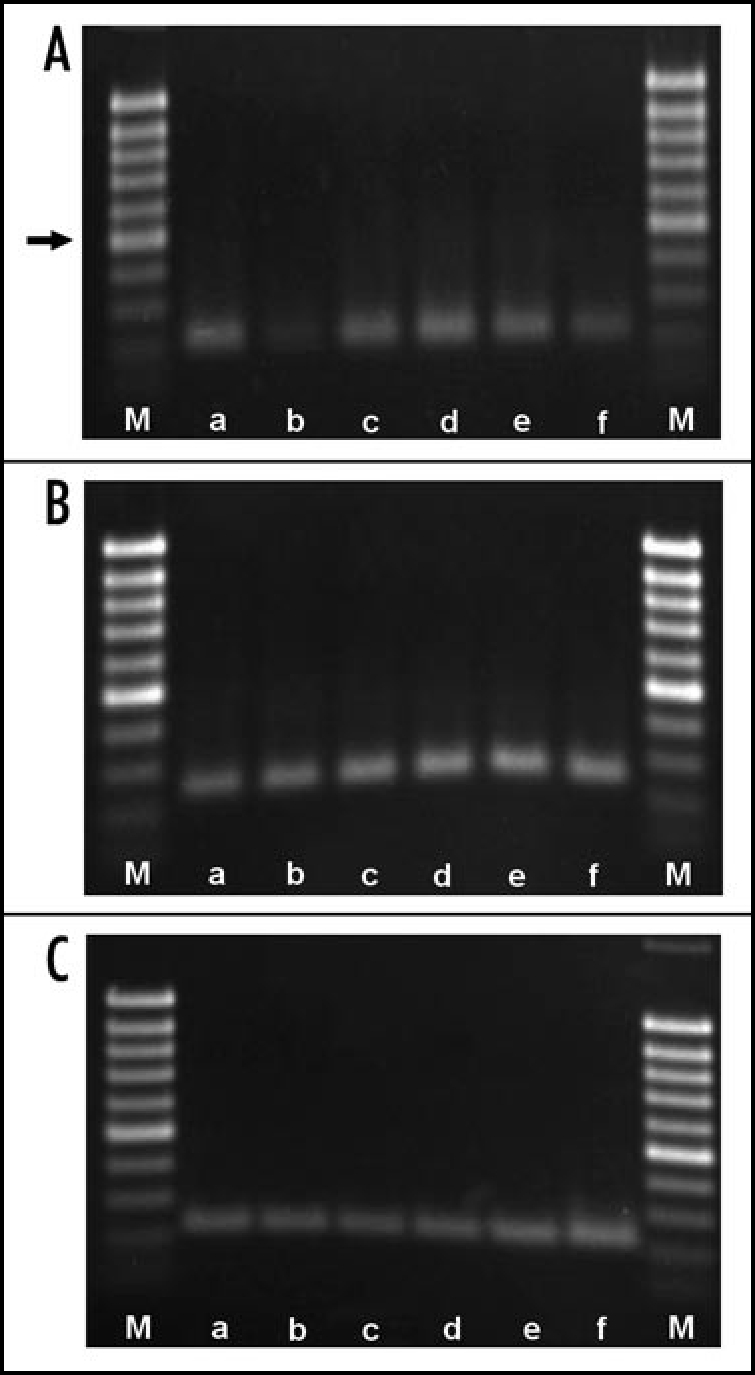
Proof of CER6 (A), actin2 (B) and Ef1α (C) transcripts in Arabidopsis thaliana rosette leaves. a, positive control; b, directly after first de-waxing; c, directly after second de-waxing; d, 1 h after second de-waxing; e, 2 h after second de-waxing; f, 3 h after second de-waxing. CER6 fragment: 212 bp, actin2 fragment: 226 bp, eF1α fragment: 250 bp. Arrow points to 500 bp marker.
A first removal of the wax layers resulted in a slight gene induction after 3.5 h (Fig. 2). A clear, strong induction was observed after a second removal with a maximum at 1.5 h after the treatment. Subsequently, the transcript level dropped dramatically during the next hours. Thus, in fully developed rosette leaves, a transient CER6 induction appears within a few hours after total removal of the wax layers. In old leaves, de-waxing initiated senescence indicated by yellowing and drying up within a few hours. Therefore, the transcript level of treated old leaves was not further investigated.
Figure 2.
CER6 transcript levels in 50 ng cDNA from rosette leaves. 1, control young rosette leaves (yrl); 2, control old rosette leaves (orl); 3, fully developed rosette leaves (fdrl), 3, 5 h after first de-waxing; 4, fdrl directly after second de-waxing; 5, fdrl 1 h; 6, fdrl 1.5 h; 7, 2 h; 8, 3.5 h after second de-waxing. 9, 10, yrl controls; 11, fdrl control; 12, yrl directly after 24 h exposure to 100/50 mg camphor/menthol; 13, yrl after 24 h exposure to 100/50 mg camphor/menthol plus 1.5 h recovery; 14, yrl directly after 24 h exposure to 50/25 mg camphor/menthol; 15, yrl after 24 h exposure to 50/25 mg camphor/menthol plus 1.5 h of recovery.
Transcript levels in plants treated with camphor and menthol for 24 h were determined directly and 1.5 h after exposure. The amount of transcripts did not increase and was similar as found in the controls (Fig. 2). CER6 expression was not monitored later as prolonged exposure to the monoterpenes was deleterious to the plants. They dried up even when watering was optimal (Fig. 3C).
Figure 3.
Effects of camphor and menthol on Arabidopsis thaliana plants. (A) growth stimulation (two plants left) in comparison to control plants (two plants right) after 24 h exposure to 50/25 mg Camphor/menthol. Growth stimulation became visible 2–3 days after the treatment. (B and C) start of (B, black arrow) and complete desiccation (C) after long term exposure to 100/50 mg camphor/menthol. Arrows point to yrl and orl as used for transcript level studies (Fig. 2) and to a leaf where desiccation started. (D) necrotic spots on leaf (arrow) two days after 24 h exposure to 100/50 mg camphor/menthol.
Induction of CER6 protein.
Polyclonal antibodies raised against the N-terminal CER6 protein fragment recognized a band in the range of 60 kD. This protein is supposed to present the full length CER6 protein. Several other bands were weakly stained in the range of 45 and 20 kD. In fully developed leaves, only a very weak or no immunoresponse was found as well as directly after de-waxing (Fig. 4A). A faint response was present 2 h (two treatments) and a relatively strong one 4 h after de-waxing. Thus, appearance of the translation product follows gene induction with a delay of 0.5 to 2.5 h. 17.5 h after de-waxing the band was almost not detectable. The results indicate that in fully developed leaves CER6 protein is very low in abundance. Gene induction by de-waxing initiated de novo synthesis of the protein, but the existence of the protein is transient. Apparently, during this physiological stage of the leaf, CER 6 is a short lived protein, only present under defined conditions where VLCFA synthesis is required.
Figure 4.
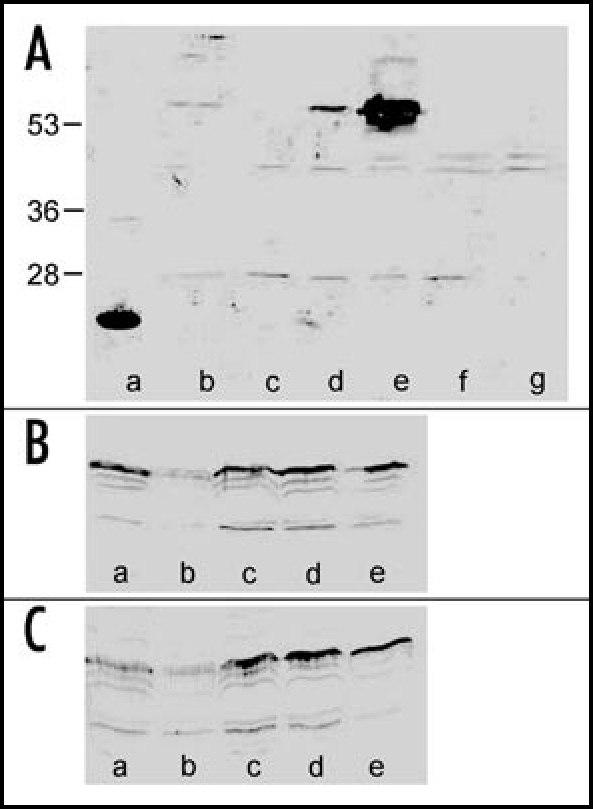
Immunostaining of CER6 protein in protein extracts of Arabidopsis thaliana. (A) a: CER6 fragment (positive control); b protein from fully developed leaves, control; c: directly after second de-waxing; d: 2 h after second de-waxing; e: 4 h after second de-waxing; f,g: 17,5 h after second de-waxing. Arrow marks position of full length CER6 protein. Numbers indicate marker proteins (kDa). (B) controls; a: flower; b: stem; c: upper stem leaves; d: lower stem leaves; e: young rosette leaves. (C) samples from plants after 24 h exposure to 100/50 mg camphor/menthol, a–e same plant parts as shown in blot B.
In young leaves, stems and flowers an immunoreactive band of 60 kD was always detectable. (Fig. 4B and C). Again, several immunoresponses were found with a stronger staining than observed in fully developed, older leaves. Monoterpene treatment did not result in a clear increase of the CER6 protein level which is consistent with the data obtained by real time PCR. In samples from flowers, a number of proteins were stained in the high molecular weight range (data not shown). There was no response in this range with samples obtained from the controls. Additional immunoresponses are thought to be due to some reactivity of the antibody to other VLCFAs or they present degradation products of CER6.
Transpiration rates after wax removal and monoterpene exposure.
The desiccation of plants after prolonged exposure to monoterpenes prompted us to check the transpiration rate of the leaves after 24 h of exposure in comparison to de-waxed and control leaves. The transpiration rate of control leaves was determined to be 3.34 ± 0.63 mmol / m2 s−1. Removal of the waxes resulted in an average transpiration rate of 5.2 ± 0.8 mmol m2 s −1 and after monoterpene treatment a value of 4.9 ± 1.0 mmol m2 s−1 was found. Thus, both treatments resulted in a drastic increase of transpiration (+ 55.6 and + 46.7%, respectively), explaining the desiccation of older leaves after de-waxing and of whole plants after monoterpene exposure. However, there were also leaves that recover from monoterpene treatment after several hours, as indicated by a decrease of the transpiration rate to the control level. Recovery of the transpiration rate to the control level was also observed with de-waxed leaves after one day.
Microscopical studies and GC analysis.
Whereas desiccation of old leaves after removal of surface waxes is in agreement with published data,16 drying up of whole plants after prolonged monoterpene treatment needed further investigations.
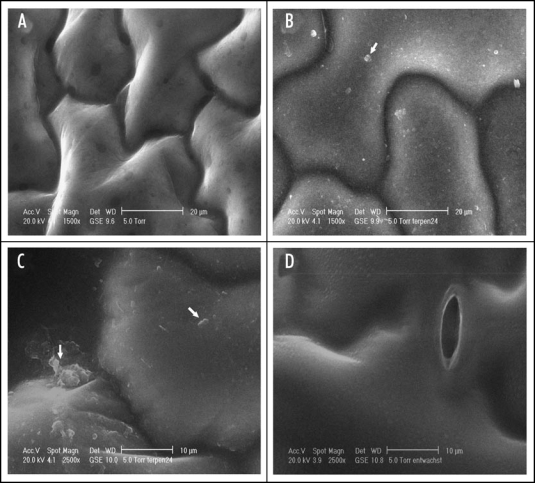
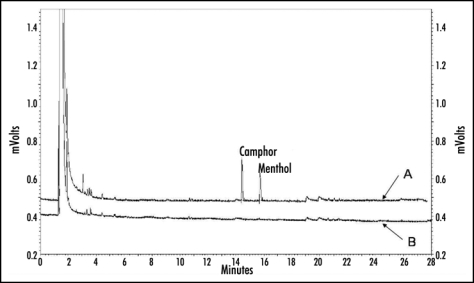
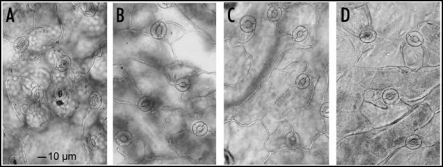
Therefore, exposed plants were studied microscopically. De-waxed leaves were also investigated to check whether de-waxing injured epidermal cells or resulted in cracks of the leaf surface. According to the microscopical check de-waxed leaves, showing a dull surface in contrast to the controls, did not exhibit damaged epidermal cells or ruptures (Fig. 5). The surface of leaves exposed to monoterpens shows some spots with platelet-like structures. In some cases these structures seemed to be fused with the surface layers of the leaves. Wax crystals could not be observed, which is in agreement with the findings of other investigators.31 Neither the surface of the control nor that of de-waxed leaves showed similar structures. It was assumed that the platelet like structures present condensed monoterpenes mixed up with cuticular waxes. The structures disappeared after several hours when plants were transferred to monoterpene free air, indicating an evaporation of the assumed condensed monoterpenes from the leaf surfaces. GC analysis were performed to ascertain, whether camphor and menthol can be found at the leaf surface. The analyses clearly showed that monoterpene exposed leaves contain the two compounds, whereas samples taken from control plants did not (Fig. 6). The platelet like structures found on leaf surfaces resulted from monoterpene treatment. They may contain condensed camphor and menthol molecules which were trapped by the hydrophobic epicuticular wax layer.
Figure 5.
ESEM of Arabidopsis leaves exposed to camphor and menthol in comparison to de-waxed and control leaves. (A) control; (B and C) platelet like structures appear on the epidermal cell surface after exposure to the monoterpenes (arrows point to the characteristic structures which are absent from the control and the surface of a de-waxed leaf (D).
Figure 6.
GC-chromatogram of dichlormethane extracts from (yrl + orl) control leaves (B) and (yrl + orl) leaves exposed to the monoterpenes (A). Camphor and menthol were only present in samples obtained from leaves after the exposure.
Effects of monoterpenes on stomatal aperture.
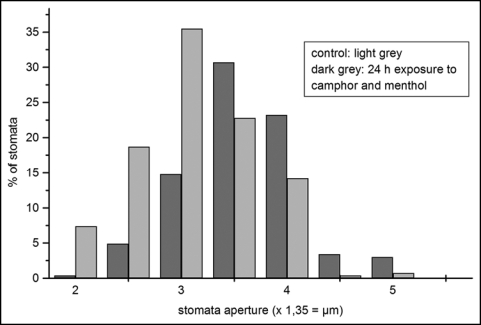
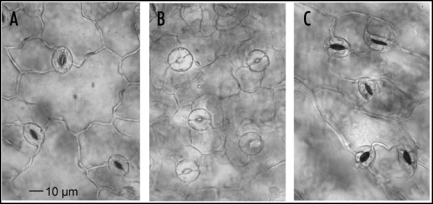
Although the wax layer was definitely affected by the monoterpenes, an increase of the transpiration rate can also be caused by injury of the stomata. Light microscopical studies revealed a higher percentage of larger stomata apertures after 24 h of monoterpene exposure (Fig. 7). Whereas most of the apertures of the control plants were between 3.37 and 4.72 µm, the majority of the apertures of the plants exposed to monoterpenes were between 4.72 and 5.4 µm. The effects were intensified during the next 24 h. After the 48 h treatments, most of the guard cells exhibited a balloon-like shape, but after 72 h, a considerable part was collapsed as indicated by coagulated cell contents (Fig. 8). Longer exposures resulted in an increase of destroyed stomata and drying up of the plants (Fig. 3B and C).
Figure 7.
Size distribution of stomata apertures of control and monoterpene treated leaves after 24 h. Control: n = 274; treated: n = 266. Treatment effect was highly significant (p = < 0.001, T-test). Stomata aperture: 2 = 2,7 µm; 3 = 4,05 µm; 4 = 5,4 µm; 5 = 6,75 µm.
Figure 8.
Stomata apertures of control (A), after 48 h (B) of exposure to camphor and menthol (100 / 50 mg) and after 72 h (C) of exposure. (B) illustrates the balloon-like shape of the stomata cells and a wider opening of the pores. (C) shows a severe damage of the stomata cells with break down of protoplasts.
Monoterpene exposure: necrotic spots or growth stimulation.
Whereas long term exposure (96 h) to 100 mg camphor/ 50 mg menthol killed the plants by desiccation, a 24 h treatment had no visible effects directly after exposure and during the following hours. However, within the next 24–48 h necrotic spots developed all over the leaf surface (Fig. 3D). Clearly, within these areas no regeneration of the wax layer can occur. However, 24 h exposure to 50 mg camphor and 25 mg menthol could also stimulate plant growth (Fig. 3A). The different reactions of the plants point to a dose and time dependence of effects caused by the monoterpenes as it is known for other allelochemicals.32
Effects of volatiles from Lavandula latifolia, Artemisia camphorata and Mentha piperita on stomatal aperture.
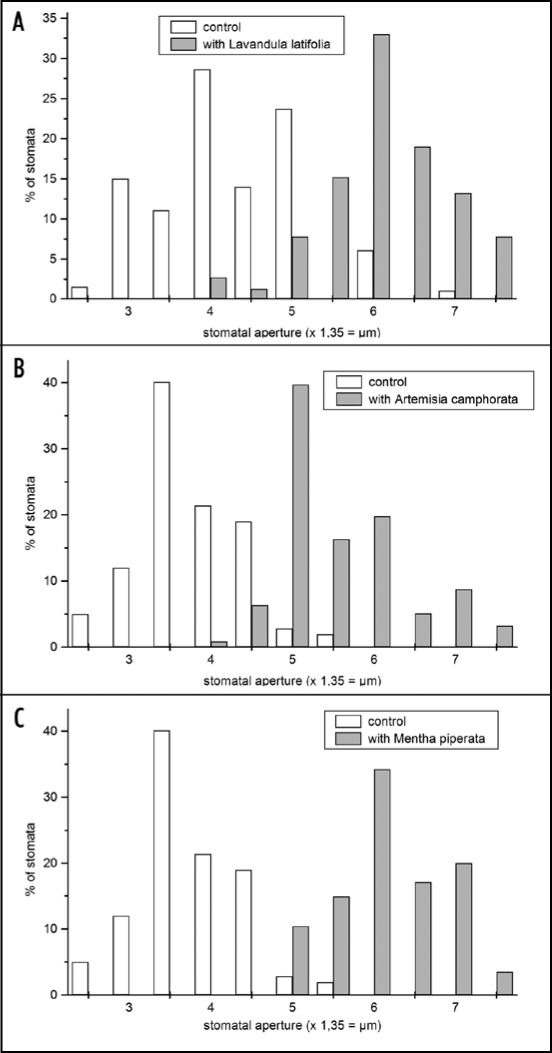
The effects of only two combined monoterpenes do not simulate natural conditions. Emitted volatiles from plants are combinations of numerous compounds including isoprene, monoterpenes and sesquiterpenes.33,34 In order to design a more natural situation, Arabidosis plants were exposed to volatiles of Lavandula latifolia, Artemisia camphorata (a considerable fraction of the essential oils of both plants is camphor) or Mentha piperita with menthol as a characteristic constituent. The presence of all three species caused stomatal opening in Arabidopsis leaves after 24 h to a greater extent as it was previously observed with camphor and menthol (Figs. 9 and 10) and shifting to larger pores was dramatic compared to the controls. However, the stomata were less damaged after 48 h than observed with camphor and menthol only which point to a different mode of action of volatile mixtures.
Figure 9.
Size distribution of stomata apertures of control leaves and leaves exposed to volatiles of Lavandula latifolia (A), Artemisia camphorata (B) and Mentha piperata (C). (A) control: n = 298; Lavandula latifolia: n = 271. (B) control: n = 162; Artemisia camphorata: n = 384; (C) control: n = 264, Mentha piperata n = 442. Treatment effects were highly significant with all species (p = < 0.001, t-test). Stomata aperture: 2 = 2.7 µm; 3 = 4.05 µm; 4 = 5.4 µm; 5 = 6.75 µm; 6 = 8.1 µm; 7 = 9.45 µm.
Discussion
The concentrations of monoterpenes used in this study are higher than the ones realized in nature. This may be true also for the volatiles of the aromatic species used in our study, since under natural conditions, the concentration of the compounds is diluted by air movements. Ciccioli et al. reported on terpene emission rates for orange trees ranging from 10 ng m−2 (leaf area) s−1 to 100 ng m−2 s−1 at 30°C and at 1000 µmol m−2 s−1 of photon flux, depending on Citrus varieties.35 The terpene mixture released by the trees during July contained 70% β-caryophyllene, thus 25.2–252 µg β-caryophyllene in 1 h or 6.05 mg in 24 h was emitted per m−2 leaf. Monoterpene volatilization differs highly between species and varieties, depends on plant age and organ, on light intensity and quality, on temperature and humidity in addition to other parameters.34,36–38 This indicates a tremendous variation in the concentrations of monoterpenes, ranging from ng up to mg/leaf area (m2) and 24 h, which may affect plants growing in the neighborhood of volatile donor plants. However, there is no knowledge available about amounts of volatiles which will indeed hit the surface of an acceptor plant. The amounts can be very different from those which are emitted by the donor plants due to air movements and whether acceptor plants grow windward of the donor plant or not. Therefore, emitted amounts of volatiles give only limited information of doses that really affect acceptor plants.
Allelopathic effects of monoterpenes on acceptor plant surface and stomata are hardly investigated, in contrast to voltatile composition and their emission of donor plants. In our study, we started therefore with rather high concentrations of campher and menthol to pinpoint possible effects. Considering published data, the concentration of volatiles emitted by the aromatic plants should be much lower (Mentha piperata: 118.8 µg m−2 /24 h; Salvia mellifera: 3.14–0.1 mg per excised branch/ 24 h).37,39 Even so, the stomata of Arabidopsis thaliana were highly respondent and pores larger than observed with camphor and menthol only.
The results presented clearly indicate deleterious effects of the monoterpenes on cuticular wax layers. The compounds prevent stomatal closure and induced swelling or even disruption of the guard cells. Both effects act in a synergistic way that enhanced transpiration and desiccation of the plants after 72 h or longer exposure. Condensed monoterpenes on the leaf surface are not only sites where a direct contact of the compounds with the cells below take place but they are as well local sources of a constant production of a concentrated monoterpene vapor that can diffuse via stomatal openings into the inner parts of the leaf. Such events are assumed to initiate membrane deteriorations, consistent with the balloon like shape of the guard cells, and a break down not only of guard cell protoplasts but also of mesophyll cells.
The monoterpenes obviously interfered with stomatal movement. At present it is not clear whether opening of the aperture initiated from starting disorders in guard cell functions or whether opening was also part of an early response belonging to VOC-mediated plant-plant conversations that appear after herbivore attack.40,41 In this case the mechanism is abused by plants emitting volatile allelochemicals like monoterpenes that hit acceptor plants with toxic amounts.
Volatiles from Lavandula latifolia, Artemisia camphorata and Mentha piperita induced a wider opening of the stomata with less swollen protoplasts than camphor and menthol, indicating a higher efficiency in stomata opening of volatile compound bouquets emitted by these species, although during long term exposure in the exsiccators, aromas of the aromatic plants became weaker. Arabidopsis stomata were less damaged over time than observed after treatment with a mixture of camphor and menthol. In addition, Arabidopsis plants did not dry up. It is known for many plants that volatile emission follow diurnal rhythms with variations in the monoterpene composition.34,35,42,43 In this case the allelopathic potential of aromatic plants can change over the day, may address different cellular targets and even different acceptor plants dependent on day time. Stomata opening by volatiles may be a general effect since it was also observed with coffee plants exposed to Mentha piperita, Ocimum basilicum, Salvia officinalis or Origanum vulgare and with cabbage exposed to camphor and menthol (Schulz et al., unpublished).
In continuing studies concentration thresholds necessary to initiate stomata opening as well as the efficiency of single compounds and combinations of them will be elucidated.
In contrast to the mechanical removal of the wax layers, expression of the CER6 gene was not higher after exposure to the monoterpenes than found in the controls. However, there was already a relatively high abundance of the protein in younger leaves. Thus, the prerequisites allowing repair mechanisms were given, despite no upregulation of the gene expression was found after the evaluated time of exposure. In this study we did not check a possible gene induction after short term exposures. It is therefore possible that an upregulation happened much earlier and the induction was already downregulated after 24 h. It is unknown if monoterpenes can alter properties of the wax layer constantly even when they have been evaporated again from the leaf surface, or if the species-dependent wax composition and the thickness of wax layers influence wax-monoterpene chemical interactions.
Short term exposure of Arabidopsis to monoterpenes can apparently result in the opposite effect, in growth stimulation. Effects on biomass allocation and an increase of root biomass by volatiles was observed in barley.44,45 Thus, under certain circumstances, positive influences of volatiles on plant growth can appear. We are currently investigating molecular events, due to monoterpene influences, that may be responsible for growth stimulations. This includes a possible higher rate of CO2 fixation.
Harmful effects of monoterpenes can be caused not only by a relatively short exposure to a more concentrated vapor of the monoterpenes but also by long term exposure to low concentrations. It is known that the reaction of plants to any allelochemical depends on the species and dose applied over time, but is also influenced by additional parameters such as biotic and abiotic stress and plant fitness.46,47 Therefore, a long term exposure to relatively low concentrations may result in a damage of plants neighbored by monoterpene emitting species. From our results is concluded that the cuticle and the stomata, resulting in enhanced transpiration, are affected first when plants are exposed to allelopathic monoterpenes.
Figure 10.
Stomata apertures of control (A) and after 24 h exposure to volatiles of (B) Artemisia camphorata, (C) Lavandula latifolia and (C) Mentha piperata. Volatiles of all three species induced strong stomata opening but stomata cells were not puffed up as observed with camphor and menthol. Apertures were larger than observed after exposure to the monoterpene mixture.
Acknowledgements
M. Schulz thanks the University of Bonn and the Faculty of Agronomy for financial support (PRIOR, project A1). We thank Prof. Schellander and his coworkers for support with the ABI Prism 7000 SDS instrument equipment.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4469
References
- 1.Muller CH. The role of chemical inhibition (allelopathy) in vegetational composition. Bull Torr Bot Club. 1966;93:332–351. [Google Scholar]
- 2.Muller WH, Muller CH. Volatile growth inhibitors produced by Salvia species. Bull Torr Bot Club. 1964;91:327–330. [Google Scholar]
- 3.Muller WH, Hague R. Volatile growth inhibitors produced by Salvia leucophylla: Effect on seedling anatomy. Bull Torr Bot Club. 1967;94:182–191. [Google Scholar]
- 4.Richardson DR, Williamson GB. Allelopathic effects of shrubs of the sand pine scrub on pines and grasses of the sand hills. For Sci. 1988;34:592–605. [Google Scholar]
- 5.Vokou D, Douvli P, Blionis GJ, Halley JM. Effects of monoterpenoids, acting alone or in pairs, on seed germination and subsequent seedlings growth. J Chem Ecol. 2003;29:2281–2301. doi: 10.1023/a:1026274430898. [DOI] [PubMed] [Google Scholar]
- 6.Barney JN, Hay AG, Weston LA. Isolation and characterization of allelopathic volatiles from mudwort (Artemisia vulgaris) J Chem Ecol. 2005;31:247–265. doi: 10.1007/s10886-005-1339-8. [DOI] [PubMed] [Google Scholar]
- 7.Weidenhamer JD, Macias FA, Fischer NH, Williamson GB. Just how insoluble are monoterpenes? J Chem Ecol. 1993;19:1827–1835. doi: 10.1007/BF00982309. [DOI] [PubMed] [Google Scholar]
- 8.Duke SO, Oliva A. Mode of action of phytotoxic terpenoids. In: Macias FA, Galindo JCG, Molinillo JMG, Cutler HG, editors. Allelopathy, Chemistry and Mode of Action of Allelochemicals. Boca Raton: CRC Press; 2004. [Google Scholar]
- 9.Dayan FE, Romagni JG, Duke SO. Investigation the mode of action of natural phytotoxins. J Chem Ecol. 2000;26:2079–2094. [Google Scholar]
- 10.Romagni JG, Allen SN, Dayan FE. Allelopathic effects of volatile cineols on two weedy plant species. J Chem Ecol. 2000;26:303–313. [Google Scholar]
- 11.Romagni JG, Duke SO, Dayan FE. Inhibition of plant asparagine synthetase by monoterpene cineoles. Plant Physiol. 2000;123:725–732. doi: 10.1104/pp.123.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Fischer NH. The function of mono and sesquiterpenes as plant germination and growth regulators. In: Putnam AR, Tang CS, editors. The Science of Allelopathy. New York: John Wiley and Sons; 1986. [Google Scholar]
- 13.Riederer M, Schreiber L. Protecting against water loss: Analysis of the barrier properties of plant cuticles. J Exp Bot. 2001;52:2023–2032. doi: 10.1093/jexbot/52.363.2023. [DOI] [PubMed] [Google Scholar]
- 14.Percy KE, Riding RT. The epicuticular waxes of Pinus strobus subjected to air pollutants. Can J For Res. 1978;8:474–477. [Google Scholar]
- 15.Long LM, Patel P, Cory WC, Steoleton AE. The maize epicuticular wax layer provides UV protection. Function Plant Biol. 2003;30:75–81. doi: 10.1071/FP02159. [DOI] [PubMed] [Google Scholar]
- 16.Todd J, Post-Beittenmiller D, Jaworsky JG. KCS1 encodes a fatty acid elongase 3-ketoacyl-CoA synthase affecting wax biosynthesis in Arabidopsis thaliana. Plant J. 1999;17:119–130. doi: 10.1046/j.1365-313x.1999.00352.x. [DOI] [PubMed] [Google Scholar]
- 17.Yephemov A, Wisman E, Huijer P, Huijse RC, Wellesen K, Saedler H. Characterization of the FIDDLEHEAD gene of Arabidopsis reveals a link between adhesion response and cell differentiation in the epidermis. Plant Cell. 1999;11:2187–2201. doi: 10.1105/tpc.11.11.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millar AA, Clemens S, Zachgo S, Giblin EM, Taylor DC, Kunst L. CUT1, an Arabidopsis gene required for cuticular wax biosynthesis and pollen fertility, encodes a very-long-chain fatty acid condensing enzyme. Plant Cell. 1999;11:825–838. doi: 10.1105/tpc.11.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pruitt RE, Vielle-Calzada JP, Ploense SE, Grossnikolaus U, Lolle SJ. Fiddelhead, a gene required to supress epidermal cell interactions in Arabidopsis encodes a putative lipid biosynthetic enzyme. Proc Natl Acad Sci USA. 2000;97:1311–1316. doi: 10.1073/pnas.97.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiebig A, Mayfield JA, Miley NL, Chau S, Fischer RL, Preuss D. Alterations in CER6, a gene identical to CUT1, differently affect longchain lipid content on the surface of pollen and stems. Plant Cell. 2000;12:2001–2008. doi: 10.1105/tpc.12.10.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hooker TS, Millar AA, Kunst L. Significance of the expression of the CER6 condensing enzyme for cuticular wax production in Arabidopsis. Plant Physiol. 2002;129:1568–1580. doi: 10.1104/pp.003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Post-Beittenmiller D. Biochemistry and molecular biology of wax production in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:405–430. doi: 10.1146/annurev.arplant.47.1.405. [DOI] [PubMed] [Google Scholar]
- 23.Jenks MA, Eigenbrode SD, Lemieux B. Cuticular waxes of Arabidopsis. In: Somerville CR, Meyerowitz EM, editors. The Arabidopsis Book. Rockville, MD: American Society of Plant Biologists; www.aspb.org/publications/arabidopsis/2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pighin JA, Zheng H, Balakshin LJ, Goodman JP, Western TL, Jetter R, Kunst L, Samuels AL. Plant cuticular lipid export requires an ABC transporter. Science. 2004;306:702–704. doi: 10.1126/science.1102331. [DOI] [PubMed] [Google Scholar]
- 25.Schulz B, Frommer WB. A plant ABC transporter takes the Lotus seat. Science. 2004;306:622–625. doi: 10.1126/science.1105227. [DOI] [PubMed] [Google Scholar]
- 26.Neinhuis C, Koch K, Barthlott W. Movement and regeneration of epicuticular waxes through plant cuticles. Planta. 2001;213:427–434. doi: 10.1007/s004250100530. [DOI] [PubMed] [Google Scholar]
- 27.El-Halawany N, Ponsuksili S, Wimmers K, Gilles M, Tesfaye D, Schellander K. Quantitative expression analysis of blastocyst-derived gene transcripts in preimplantation developmental stages of the in vitro-produced bovine embryos using real-time polymerase chain reaction technology. Reprod Fert Develop. 2004;16:753–762. doi: 10.1071/rd04041. [DOI] [PubMed] [Google Scholar]
- 28.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Molec Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 29.Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): Trends and problems. J Molec Endocrinol. 2002;29:23–29. doi: 10.1677/jme.0.0290023. [DOI] [PubMed] [Google Scholar]
- 30.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenks MA, Tuttle HA, Eigenbrode SD, Feldmann A. Leaf epicuticular waxes of the Eceriferum mutants in Arabidopsis. Plant Physiol. 1995;108:369–377. doi: 10.1104/pp.108.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulz M, Friebe A. Detoxification of allelochemicals in higher plants and enzymes involved. In: Inderjit Dakshini KMM, Foy CL, editors. Principles and Practices in Plant Ecology. Allelochemical Interactions. Boca Raton USA: CRC Press LLC; 1999. pp. 383–400. [Google Scholar]
- 33.Sharkey TD, Yeh SS. Isoprene emission from plants. Annu Rev Plant Physiol Mol Biol. 1995;52:407–436. doi: 10.1146/annurev.arplant.52.1.407. [DOI] [PubMed] [Google Scholar]
- 34.Kesselmeier J, Staudt M. Biogenic volatile organic compouds (VOC): An overview on emission, physiology and ecology. J Atmosph Chem. 1999;33:23–88. [Google Scholar]
- 35.Ciccioli P, Brancaleoni E, Frattoni M, Di Palo V, Valentini R, Tirone G, Seufert G, Bertin N, Hansen U, Csiky O, Lenz R, Sharma M. Emission of reactive terpene compounds from orange orchards and their removal by within canopy processes. J Geophys Res. 104:8077–8094. [Google Scholar]
- 36.Martin DM, Gershenzon J, Bohlmann J. Induction of volatile terpene biosynthesis and diurnal emission by methyl jasmonate in foliage of Norway spruce. Plant Physiol. 2003;132:1586–1599. doi: 10.1104/pp.103.021196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dement WA, Tyson EJ, Mooney HA. Mechanism of monoterpene volatilization in Salvia mellifera. Phytochemistry. 1975;14:2555–2557. [Google Scholar]
- 38.Street RA, Owen S, Duckham SC, Boissard C, Hewitt CN. Effect of habitat and age in volatile organic compound (VOC) emission from Quercus ilex and Pinus pinea. Atmos environ. 1997;31:89–100. [Google Scholar]
- 39.Gershenzon J, McConkey, Croteau RB. Regulation of monoterpene accumulation in leaves of peppermint. Plant Physiol. 2000;122:205–213. doi: 10.1104/pp.122.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baldwin IT, Halitschke R, Paschold A, von Dahl CC, Preston CA. Volatile signaling in plant-plant interactions:“talking trees“ in the genomics era. Science. 2006;311:812–815. doi: 10.1126/science.1118446. [DOI] [PubMed] [Google Scholar]
- 41.Koch T, Krumm T, Jung V, Engelberth J, Boland W. Differential induction of plant volatile biosynthesis in the lima bean by early and late intermediates of the octadecanoid -signaling pathway. Plant Physiol. 1999;121:153–162. doi: 10.1104/pp.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bufler U, Wegmann K. Diurnal variation of monoterpne concentrations in open-top chambers and in the Welzheim forest air. F.R.G. Atmosph Environ. 1991;25A:251–256. [Google Scholar]
- 43.Farmer EE. Surface to air signals. Nature. 2001;411:854–856. doi: 10.1038/35081189. [DOI] [PubMed] [Google Scholar]
- 44.Ninkovic V. Volatile communication between barley plants affects biomass allocation. J Exp Bot. 2003;54:1931–1939. doi: 10.1093/jxb/erg192. [DOI] [PubMed] [Google Scholar]
- 45.Ninkov V, Glinwood R, Pettersson J. Communication between undamaged plants by volatiles: The role of allelobiosis. In: BaluŠka F, Mancuso S, Volkmann D, editors. Communication in plants. Springer, Berlin: Neuronal aspects of plant life; 2006. [Google Scholar]
- 46.Sicker D, Schneider B, Hennig L, Knop M, Schulz M. Glucoside carbamates from benzoxazolin-2(3H)-one detoxification in extracts and exudates of corn roots. Phytochemisty. 2001;58:819–925. doi: 10.1016/s0031-9422(01)00299-0. [DOI] [PubMed] [Google Scholar]
- 47.Knop M, Pacyna S, Voloshchuk N, Kant S, Müllenborn C, Steiner U, Kirchmair M, Scherer W, Schulz M. Zea mays: Benzoxazolinone detoxification under sulfur deficiency conditions-A complex allelopathic alliance including endophytic Fusarium verticillioides. J Chem Ecol. 2007;33:225–237. doi: 10.1007/s10886-006-9226-5. [DOI] [PubMed] [Google Scholar]