Abstract
Auxin (IAA) is versatile signalling molecule of plants, currently classified as plant hormone. But there are data suggesting that auxin is acting also as plant-specific morphogen, electric-responses inducing transmitter, and as general signalling molecule used for plant-bacteria communication. Our previous data revealed that auxin is associated with secretory endosomes and also highly enriched within cell walls of cells active in transcellular auxin transport. Our present data, based on in vivo non-invasive auxin flux recordings, reveal that auxin is secreted out of synaptic-like domains specialized for efflux of auxin in root apex cells highly active in polar cell-cell transport of auxin. We obtained both genetic and pharmacological evidence that phospholipase Dζ2 drives vesicular secretion of auxin for its polar transcellular transport in the transition zone of the root apex. Secretion of auxin via secretory vesicles has far-reaching consequences not only for our understanding of cell-cell auxin transport but also for plant sciences as a whole.
Key Words: auxin, Brefeldin A, plant synapse, phospholipase D, phosphatidic acid, secretion, vesicle recycling
Introduction
Polar transport of auxin (IAA) is a characteristic feature of almost all plant cells which are inherently polarized into the auxin-influx (input) pole and the auxin-efflux (output) pole. Ever since Charles Darwin predicted1 and Fritz Went discovered2 polar transport of auxin as the principal mechanism underlying plant polarity and organ movements, numerous papers were published on this most important phenomenon in plant sciences. Early papers revealed that auxin transport is an active process requiring continuous respiration, supply of cellular energy in form of ATP, and physiological temperatures.3–5 Here we provide in vivo pharmacological and genetic evidence that substantial portion of the polar transport of auxin in root apices is driven by vesicle-mediated secretion regulated by the PLDζ2 activity and its product PA. In the PLD2 mutant and after 1-butanol treatment, auxin fluxes measured by the IAA-sensitive microelectrode are strongly supressed despite undisturbed PINs localization.
From almost 35 years old study we know that centrifugal displacement of cytoplasm either stimulates efflux of auxin, if cytoplasm accumulates at the auxin-efflux pole, or has just the opposite effect if it accumulates at the auxin-influx pole.6 This interesting finding supported even older observations by Rainer Hertel, Helen Goldsmith and their colleagues that auxin-efflux pole is stimulated in polar auxin secretion both via sedimented protoplast aided by the sedimented statoliths and via mechanically-induced centrifugal relocation of cytoplasm.3,7 Together with tight dependence of the polar auxin transport on continuous energy supply and physiological temperature, these and other findings resulted eventually in the secretory theory of polar auxin transport. Unfortunately, this secretory theory was only vaguely articulated and was eventually replaced by the chemiosmotic theory of polar auxin diffusion in seventies of the last century.8,9 Although the ‘passive’ chemiosmotic theory was often presented as an alternative to the ‘active’ polar secretory theory of auxin transport,9 both theories, in fact, deal with different aspects of the polar auxin transport. The secretory theory deals with the active auxin efflux whereas the chemiosmotic theory deals mainly with the passive auxin influx due to lipophilic weak acid nature of auxin and pH differences between the cytoplasm and cell wall. Ever since the polar auxin transport was shown to be dependent on active respiration and continuous ATP supply,4–6 with the efflux being the decisive process, auxin efflux carriers were postulated and looked for.8,9
Recently, manipulation of secretory activities, via phospholipase Dζ2 mutant and over-expressing lines10 as well as its signalling product phosphatidic acid (PA), supported our earlier auxin immunolocalization data11 that the vesicular recycling and secretion, and not just the bare presence of PINs at the plasma membrane, are essential for polar cell-cell auxin transport.11,12 Here we have accomplished further analysis of these mutant and transgenic plants and obtained the ultimate evidences of the essential roles of vesicular secretion for the polar transport of auxin in root apices of Arabidopsis. Using non-invasive method12 allowing sensitive and precise in vivo recordings of auxin transport in growing root apices,12–14 we have been able to analyze auxin tranport in mutant plants with disturbed vesicle recycling-based secretion.10 Our data provide evidence for vesicular secretion of auxin in root apices of Arabidopsis.
Materials and Methods
Plant materials and chemical reagents.
Transgenic lines containing DR5::GUS in wild-type and pldζ2 plants were decribed in our recent paper,10 T2 and T3 homozygous generations were used for GUS activity detection. Seven-day-old transgenic seedlings were treated with PA (final concentrations of 10, 20 or 50 µM), 1-butanol (0.4 or 0.8%, v/v) for 24 h or 48 h, BFA (50 µM) for 2.5 h, then washed with ddH2O. After treatment, seedlings were incubated with solution containing X-Glue (final concentration at 0.5 mg/mL) for 3 h, finally visualized using Nomarski optics on a Leica DMR microscope with a Leica DC300F digital camera after removal of chlorophyll.
PA (P9511) and BFA (B-7651) were purchased from Sigma-Aldrich. 1-Butanol (HPLC-grade) was purchased from Sinopharm Chemical Reagent. All plant materials were derived from Arabidopsis Columbia (Col-0) ecotype.
Recording of root apex auxin fluxes using an IAA-selective self-referencing microelectrode.
The procedure for the fabrication of the IAA-selective microsensor have already been reported.11,12 The microelectrodes were utilized as vibrating probes to monitor IAA fluxes in the root apex following the procedures described in our recent papers.11–14 Plants were grown in hydroponic cultures. All experiments were performed on roots 3 dag.
Microscopy of the GFP plants.
We used DR5::GFP of Arabidopsis thaliana for the investigation of auxin signalling. The seeds were surface sterilised and placed on ½ MS culture medium (for details see ref. 11) containing vitamins and 1% sucrose that was solidified by 0.8% agar. Seedlings were grown in vertical position under continuous light for three days.
Three day-old seedlings were transferred to microscopic slides that were modified into thin chambers by cover-slips. Chambers were filled with the same liquid medium but without agar and placed in sterile glass cuvettes containing the medium at a level that reached the open lower edge of the chambers. This allows free exchange of medium between chambers and the cuvette. Seedlings were grown in vertical position under continuous light for 12–24 hours. During this period the seedlings stabilised root growth and proceeded in the formation of new root hairs. For the treatments IAA (1 µM), BFA (35 µM), PA (10 µM), 1-butanol (0.4%), 3-butanol (0.4%) or a double treatment respectively were applied to this system. Imaging was done with a confocal laser scanning microscope (ICS Leica or/and C1si Nikon).
Results
PLDζ2 is expressed at a narrow band of cells in the root apex: distal part of the root apex transition zone.
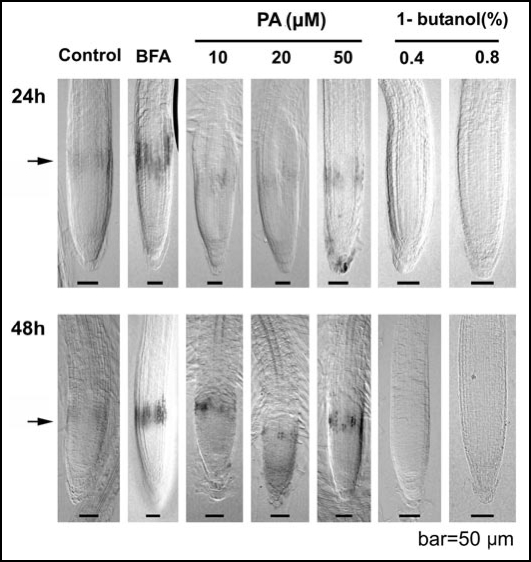
PLDζ2 is expressed in narrow band of cells (Fig. 1) corresponding to the distal portion of the transition zone (0.20–0.35 mm from the root apex).15 Brefeldin A (BFA) and phosphatidic acid (PA) treatments increase this local PLDζ2 expression, whereas inhibition of PLDζ2 activity via 1-butanol inhibits this local PLDζ2 expression in the root apex (Fig. 1).
Figure 1.
PLDζ2 expression. The PLDζ2 reporter expression in the distal portion of the transition zone increases after BFA-treatment (2,5h) and a PA-treatment whereas the reporter signal disappears after treatment with 1-butanol.
PA generated via PLD activity is essential for the auxin maximum at the distal portion of the root apex transition zone.
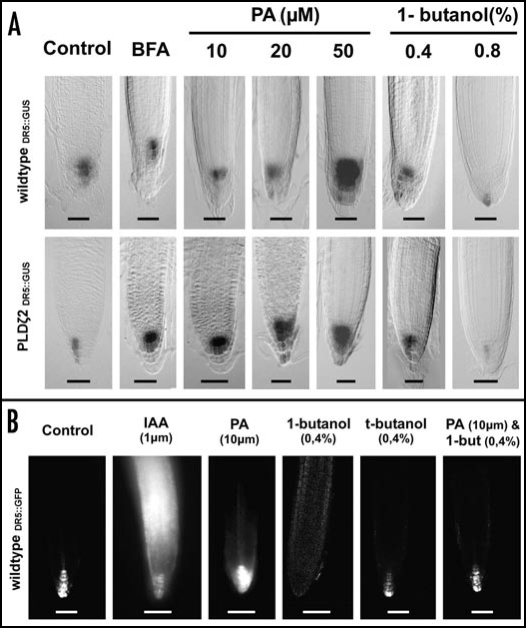
PLDζ2 activity affects the auxin maximum in the distal part of the transition zone of root apices (Fig. 2), which is the most active part from the whole plant body with respect of the polar auxin transport.16 Both DR5::GUS (Fig. 2A) and DR5::GFP (Fig. 2B) lines showed increased auxin maximum at the root tip after PA treatments and loss of this maximum after inhibition of the PLDζ2 activity with 1-butanol (Fig. 2).
Figure 2.
Auxin maximum in the root tip. (A) DR5::GUS in wildtype and PLDζ2 plants. BFA treatment for 2.5 h and PA, 1-butanol for 48 h. Bar = 50 µm. (B) With the DR5::GFP are shorter treatment times (∼4 h) sufficent to monitor changes in the transcription pattern of the DR5 reporter. Bar = 50 µm
PLDζ2 activity is responsible for vesicular secretion of auxin at the root apex transition zone.
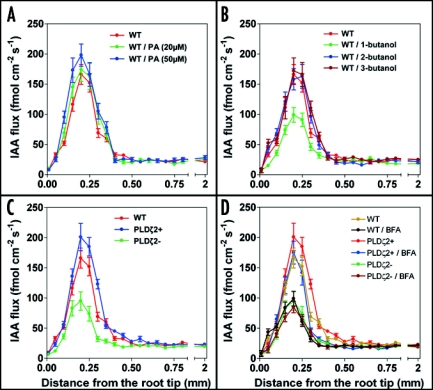
In order to assess role of PLDζ2 activity on the polar auxin transport, we have taken advantage of recently introduced new technique which can monitor auxin fluxes in intact organs of living plants.12 This technique is based on bathing of roots in well-defined solution enriched with auxin which is then taken up into endogenous streams of polar auxin transport. Using this new technique, we report here that the PLDζ2 activity is responsible for about 40% of auxin transport via vesicular secretion in the particular root apex region which we have characterized as the distal part of the transition zone of the root apex.15 Importantly, both the PLDζ2 overexpression line and PA treated roots show higher auxin transport specifically (and only) in this particular root apex region when we scored increase by about 30 % (Fig. 3A and C). In line with this, both in the pldζ2 mutant and 1-butanol-treated roots, there is of about 40% drop of the auxin transport in this root apex zone (Fig. 3B–D). Importantly, there is no change in the polar auxin transport in cells of the elongation region (Fig. 3). As brefeldin A (BFA) is not able to inhibit further the already lowered auxin transport in the PLDζ2 mutant, our data implicate that from all 12 PLDs expressed in Arabidopsis genome,17 it is particularly the PLDζ2 which is responsible for vesicular secretion of auxin at the root apex.
Figure 3.
Auxin-flux measurements in the root apex. (A) The auxin-flux in the transition zone increases proportional to a rising PA treatment. (B) The auxin-flux in the transition zone decreases only after a 1-butanol treatment. (C) The auxin-flux in the transition zone of the PLD mutants comply to the PA and 1-butanol treatments. (D) Brefeldin A (35µM) treatment decreases auxin flux in wildtype and PLDζ2 + overexpression lines but not in PLDζ2-mutant.
Our results are surprising: significant amount (about 40%) of polar auxin transport in the root apex zone with the highest rate of polar auxin transport16 and with the maximum of expression of the PLDζ2 is due to the PA-driven vesicular recycling and secretion. It emerges that only the PA produced specifically by the PLDζ2 activity is relevant in this respect.
Discussion
Active efflux (pumping out) of auxin was hypothesized to be mediated by polarly localized putative auxin carriers within the plasma membrane. About ten years ago, first of these carriers were identified in several laboratories and subsequent localization of proteins and analysis of mutants confirmed almost all expectations. Numerous papers have been published on both efflux carriers of the PIN family and influx carriers of AUX family (for recent reviews see refs. 16 and 18–21). All this was interpreted as the final evidence for the chemiosmotic theory which is generally accepted now despite several published observations which contradict several predictions of this theory.11 Importantly, this theory predicts that the localization of PINs at the plasma membrane is tightly linked with the activity of polar auxin transport. Both pharmacological and mutant approaches document that this is not the case. For instance, the powerful inhibitor of vesicular secretion brefeldin A (BFA) is well known to block the polar auxin transport within few minutes of its application despite the fact that PINs are still located and their presumptive site of action—at the plasma membrane.11 In addition, since earlier studies we also know that low temperature stops polar transport of auxin. Low temperature blocks endocytosis (for plant cells see ref. 22), locking PINs at the plasma membrane. This strongly suggest that the endocytic vesicular recycling at the plasma membrane is essential for the polar auxin transport. In fact, this dependency of auxin efflux on vesicle recycling was genetically confirmed in recent studies.23–25 In addition, the classical inhibitors of polar auxin transport, TIBA and NPA, were demasked as general inhibitors of the endocytosis-dependent vesicle recycling.23,24 As mentioned earlier, there is pharmacological and genetic evidence that the mere presence of PINs at the plasma membrane does not support full activity of the polar auxin transport.10,11
Here we have used non-invasive technique allowing sensitive and continuous monitoring of auxin fluxes at root apices. Our previous data showed convincingly that this technique monitors reliably manipulation of endogenous polar auxin streams either with classical auxin inhibitors12 or with mutants of maize11 and Arabidopsis.13,14 These endogenous auxin streams are robust enough to sustain even large sudden increases of extracellular auxin but are sensitive both to the classical auxin transport inhibitors (TIBA and NPA), recently discovered to act as inhibitors of endocytosis and vesicle recycling,23,24 and to powerful inhibitor of vesicular secretion, brefeldin A.12 Exogenous auxin fails to disturb these robust internal auxin flows but restores them in control root apices, as indicated by the recovered meristem sizes, in the pin1pin2 and pin1pin2pin7 double and triple mutants.25
These data show that specifically PLDζ2, and no other PLD from the rest of eleven PLDs expressed in Arabidopsis,17 is specialized for this vesicular regulation of the polar auxin transport in the distal portion of the transition zone in root apices. Interestingly in this respect, PLDζ2 shows similarities to animal/human PLDs.17 As brefeldin A has almost no effect on the polar auxin transport in the PLDζ2 mutant (Fig. 3D), we suggest that the BFA-sensitive auxin polar transport, driven by vesicle recycling mediated PIN1 and PIN2 cycling, needs the PLDζ2 produced PA. This conclusion has also far-reaching consequences for our understanding of the polar transport of auxin. Expression of the PLDζ2 corresponds exactly to the root apex region which is relevant for the PLDζ2/PA-driven vesicular secretion of auxin.10 This root zone expresses four PINs (1, 2, 3, 7) and three PGPs,1,4,21 the highest numbers of IAA transporters ever scored for any part of the plant body.21 It is also this root apex region in which the basipetal transport in the epidermis joins the acropetal transport in the stele.25 Previous results and our in vivo recordings show that this particular root region has by far the highest rate of auxin transport.12–14 So this specific root apex zone is unique from the polar auxin transport perspective.
There are several important conclusions in respect of the PLDζ2 activity and roles of PINs in polar auxin transport. As recycling of PINs is essential for the polar auxin transport in root apices23,24 and as recycling of both PIN210 and PIN1 (unpublished data) depends on the vesicular secretion driven by the PLDζ2 activity, the auxin transport not sensitive to manipulation of PA levels coming from the PLDζ2 activity is accomlished either via PGP-type ABC transporters which are abundantly expressed in this root apex zone13,14,21 or via small hypothetical population of non-cycling PINs transporting auxin across the plasma membrane.20 Attractive scenario remain that PINs are both auxin exporters and receptors.26
Genetic evidence and auxin immunolocalization in maize roots also strongly suggested that auxin is secreted as a cargo via recycling vesicles trafficking between secretory endosomes and polarized auxin secreting cell poles.10,11 In addition, Arabidopsis H+-pyrophosphatase VP1, which acidifies vacuoles and presumably endosomes in plant cells, was reported to control auxin transport in a similar manner27 as here reported PLDζ2. Although these authors did not mention the possibility of auxin secretion, in the commentary article to this report, hypothetical X compartment was postulated to play a role in polar auxin transport.28 Our data suggest that this X compartment might be a secretory endosome supporting auxin transport via vesicular recycling. This scenario is strongly supported not only by immunolocalization of auxin enriched within endosomes at those cross-walls (synapses) which actively transport (secrete) auxin11 but also by showing that PIN1 is abundant at plasmodesmata/pit-fields29 which are enriched with F-actin, plant-specific myosin VIII,30 and which show abundant endocytic recycling.31,32 Intriguingly in this respect, BIG protein of Arabidopsis is homologous to CALOSSIN/PUSHOVER protein of Drosophila that is involved in vesicle recycling during neuronal synaptic transmission in Drosophila,33 and is important for polar transport of auxin34 and for auxin-modulated endocytosis in plants.35
Identity of vesicular/endosomal transporters enriching auxin within endosomes of the plant endocytic network36 awaits their identification but PINs remain a hot candidates for this neurotransmitter-like vesicular loading transporters. The most attractive scenario would be that PINs act as transporters at vesicles/endosomes and as receptors at the plasma membrane. In this context, it is also possible to easily explain strong coupling observed between polar auxin transport26 and elongation growth of plant cells—both of which are direct outcome of the secretory pathway activity.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4566
References
- 1.Darwin C. The Power of Movements in Plants. London: John Murray; 1880. [Google Scholar]
- 2.Went F. Wuchsstoff und Wachstum. Rec Trav Bot Neerl. 1928;25:1–116. [Google Scholar]
- 3.Hertel R, Leopold AC. Versuche zur Analyse des Auxintransports in der Koleoptile von Zea mays L. Planta. 1963;59:535–562. [Google Scholar]
- 4.Goldsmith MHM. Separation of transit of auxin from uptake: average velocity and reversible inhibition by anaerobic conditions. Science. 1967;156:661–663. doi: 10.1126/science.156.3775.661. [DOI] [PubMed] [Google Scholar]
- 5.Wilkins MB, Martin M. Dependence of basipetal polar transport of auxin upon aerobic metabolism. Plant Physiol. 1967;42:831–839. doi: 10.1104/pp.42.6.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldsmith MHM, Ray PM. Intracellular localization of the active process in polar transport of auxin. Planta. 1973;111:297–314. doi: 10.1007/BF00385549. [DOI] [PubMed] [Google Scholar]
- 7.Ouitrakul R, Hertel R. Effect of gravity and centrifugal acceleration on auxin transport in corn coleoptiles. Planta. 1969;88:233–243. doi: 10.1007/BF00385066. [DOI] [PubMed] [Google Scholar]
- 8.Rubery PH, Sheldrake AR. Carrier-mediated auxin transport. Planta. 1974;118:101–121. doi: 10.1007/BF00388387. [DOI] [PubMed] [Google Scholar]
- 9.Goldsmith MHM. The polar transport of auxin. Ann Rev Plant Physiol. 1977;28:439–478. [Google Scholar]
- 10.Li G, Xue H-W. Arabidopsis PLDζ2 regulates vesicle trafficking and is required for auxin response. Plant Cell. 2007;19:281–295. doi: 10.1105/tpc.106.041426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlicht M, Strnad M, Scanlon MJ, Mancuso S, Hochholdinger F, Palme K, Volkmann D, Menzel D, Baluška F. Auxin immunolocalization implicates vesicular neurotransmitter-like mode of polar auxin transport in root apices. Plant Signal Behav. 2006;1:122–133. doi: 10.4161/psb.1.3.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mancuso S, Marras AM, Volker M, Baluška F. Non-invasive and continuous recordings of auxin fluxes in intact root apex with a carbon-nanotube-modified and self-referencing microelectrode. Anal Biochem. 2005;341:344–351. doi: 10.1016/j.ab.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 13.Santelia D, Vincenzetti V, Azzarello E, Bovet L, Fukao Y, Düchtig P, Mancuso S, Martinoia E, Geisler M. MDR-like ABC transporter AtPGP4 is involved in auxin-mediated lateral root and root hair development. FEBS Lett. 2006;579:5399–5406. doi: 10.1016/j.febslet.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 14.Bouchard R, Bailly A, Blakeslee JJ, Oehring SC, Vincenzetti V, Lee OR, Paponov I, Palme K, Mancuso S, Murphy AS, Schulz B, Geisler M. Immunophilin-like TWISTED DWARF1 modulates auxin efflux activities of Arabidopsis P-glycoproteins. J Biol Chem. 2006;281:30603–30612. doi: 10.1074/jbc.M604604200. [DOI] [PubMed] [Google Scholar]
- 15.Verbelen J-P, De Cnodder T, Le J, Vissenberg K, Baluška F. The root apex of Arabidopsis thaliana consists of four distinct zones of cellular activities: meristematic zone, transition zone, fast elongation zone, and growth terminating zone. Plant Signal Behav. 2006;1:296–304. doi: 10.4161/psb.1.6.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teale WD, Paponov IA, Palme K. Auxin in action: signalling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol. 2006;7:847–859. doi: 10.1038/nrm2020. [DOI] [PubMed] [Google Scholar]
- 17.Eliáš M, Potocký M, Cvrcková F, Žársky V. Molecular diversity of phospholipase D in angiosperms. BMC Genomics. 2002;3:2–16. doi: 10.1186/1471-2164-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blakeslee JJ, Peer WA, Murphy AS. Auxin transport. Curr Opin Plant Biol. 2005;8:494–500. doi: 10.1016/j.pbi.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Sieberer T, Leyser O. Auxin transport, but in which direction? Science. 2005;312:858–860. doi: 10.1126/science.1127659. [DOI] [PubMed] [Google Scholar]
- 20.Vieten A, Sauer M, Brewer PB, Friml J. Molecular and cellular aspects of auxin-transportmediated development. Trends Plant Sci. 2007;12:160–168. doi: 10.1016/j.tplants.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Bandyopadhyay A, Blakeslee JJ, Lee OR, Mravec J, Sauer M, Titapiwatanakun B, Makam SN, Bouchard R, Geisler M, Martinoia E, Friml J, Peer WA, Murphy AS. Interactions of PIN and PGP auxin transport mechanisms. Biochem Soc Trans. 2007;35:137–141. doi: 10.1042/BST0350137. [DOI] [PubMed] [Google Scholar]
- 22.Baluška F, Hlavacka A, Šamaj J, Palme K, Robinson DG, Matoh T, McCurdy DW, Menzel D, Volkmann D. F-actin-dependent endocytosis of cell wall pectins in meristematic root cells: insights from brefeldin A-induced compartments. Plant Physiol. 2002;130:422–431. doi: 10.1104/pp.007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geldner N, Friml J, Stierhof YD, Jürgens G, Palme K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature. 2001;413:425–428. doi: 10.1038/35096571. [DOI] [PubMed] [Google Scholar]
- 24.Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jürgens G. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell. 2003;112:219–230. doi: 10.1016/s0092-8674(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 25.Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- 26.Hossel D, Schmeiser C, Hertel R. Specificity patterns indicate that auxin exporters and receptors are the same proteins. Plant Biol. 2005;7:41–48. doi: 10.1055/s-2004-830475. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Yang H, Peer WA, Richter G, Blakeslee J, Bandyopadhyay A, Titapiwantakun B, Undurraga S, Khodakovskaya M, Richards EL, Krizek B, Murphy AS, Gilroy S, Gaxiola R. Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science. 2005;310:121–125. doi: 10.1126/science.1115711. [DOI] [PubMed] [Google Scholar]
- 28.Grebe M. Growth by auxin; when a weed needs acid. Science. 2005;360:60–61. doi: 10.1126/science.1119735. [DOI] [PubMed] [Google Scholar]
- 29.Zažímalová E, Krecek P, Skupa P, Hoyerová K, Petrášek J. Polar transport of the plant hormone auxin—the role pf PIN-FORMED (PIN) proteins. Cell Mol Life Sci. 2007 doi: 10.1007/s00018-007-6566-4. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Šamaj J, Chaffey NJ, Tirlapur U, Jasik J, Volkmann D, Menzel D, Baluška F. Actin and myosin VIII in plasmodesmata cell-cell channels. In: Baluška F, Volkmann D, Barlow PW, editors. Cell Cell Channels. Landes Bioscience; 2006. pp. 119–134. [Google Scholar]
- 31.Oparka KJ. Getting the message across: how do plant cells exchange macromolecular complexes? Trends Plant Sci. 2004;9:33–41. doi: 10.1016/j.tplants.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Haupt S, Cowan GH, Ziegler A, Roberts AG, Oparka KJ, Torrance L. Two plant-viral movement proteins traffic in the endocytic recycling pathway. Plant Cell. 2005;17:164–181. doi: 10.1105/tpc.104.027821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu X-ZS, Wes PD, Chen H, Li H-S, Yu M, Morgan S, Liu Y, Montell C. Retinal targets for calmodulin include proteins implicated in synaptic transmission. J Biol Chem. 1998;273:31297–31307. doi: 10.1074/jbc.273.47.31297. [DOI] [PubMed] [Google Scholar]
- 34.Gil P, Dewey E, Friml J, Zhao Y, Snowden KC, Putterill J, Palme K, Estelle M, Chory J. BIG: a calossin-like protein required for polar transport of auxin transport in Arabidopsis. Gen Dev. 2001;15:1985–1997. doi: 10.1101/gad.905201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paciorek T, Zažímalová E, Ruthardt N, Petrášek J, Stierhof YD, Kleine-Vehn J, Morris DA, Emans N, Jürgens G, Geldner N, Friml J. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature. 2005;435:1251–1256. doi: 10.1038/nature03633. [DOI] [PubMed] [Google Scholar]
- 36.Šamaj J, Read ND, Volkmann D, Menzel D, Baluška F. The endocytic network in plants. Trends Cell Biol. 2005;15:425–433. doi: 10.1016/j.tcb.2005.06.006. [DOI] [PubMed] [Google Scholar]