Abstract
The development of plants from seedlings to mature adult organisms requires the precise maintenance of the stem cell populations at meristems. In Arabidopsis, the CLAVATA (CLV) pathway is vital for homeostatic regulation of stem cell populations at shoot and floral meristems. Two components of this pathway are the protein phosphatase type 2 C proteins, POLTERGEIST (POL) and PLL1, which positively regulate stem cell specification. Recent studies by our laboratory demonstrated that POL and PLL1 are vital not only for meristem regulation but also for other essential developmental processes including those regulating the formation of the central vasculature and the basal half of the embryo. These results suggest that POL and PLL1 are components of multiple pathways, not just the CLV pathway. Furthermore, considering the processes that are disrupted in the pol pll1 double mutants it is possible that POL and PLL1 are required for certain asymmetric cell divisions during development.
Key Words: meristem, organogenesis, CLAVATA, POLTERGEIST, Arabidopsis
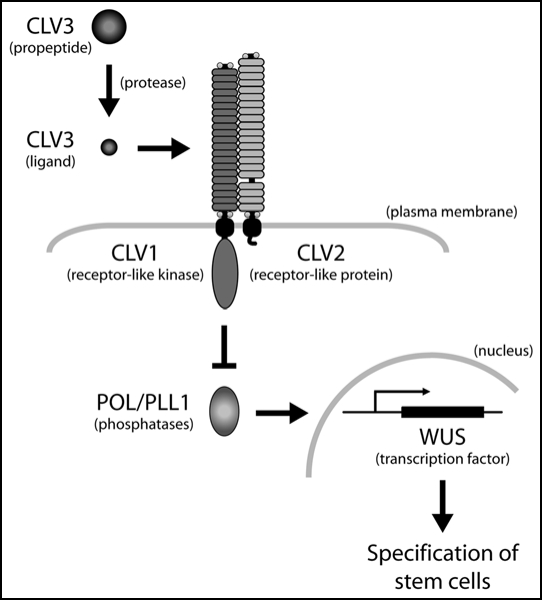
Precise regulation of plant shoot and root meristems is required for the proper development of all adult organs. Mis-regulation can lead to an over accumulation of or a lack of the stem cells needed to generate adult tissues, resulting in improper organ formation. Previous studies in Arabidopsis thaliana have identified the CLAVATA (CLV) pathway as a key regulator of meristem size.1,2 Specifically, the CLV pathway modulates the mRNA levels of the homeodomain transcription factor, WUSCHEL (WUS), which is required for the maintenance of stem cells (Fig. 1). Three components of the pathway are CLV1, CLV2, and CLV3, which encode a receptor-like kinase, a receptor-like protein and a putative ligand, respectively. These proteins are negative regulators of WUS expression and thus limit the number of stem cells. More recently, our laboratory has identified two related protein phosphatase type 2C (PP2C) proteins, POLTERGEIST (POL) and PLL1, as positive regulators of WUS expression and stem cell specification that function downstream of the CLV proteins.3–5 Our latest study in Development by Song et al, demonstrates the importance of these cellular regulators in many aspects of plant development through the analysis of pol pll1 double mutants.6
Figure 1.
Clavata pathway model. The receptor-like kinase protein, CLV1, and the receptor-like protein, CLV2, are localized to the plasma membrane. The putative ligand, CLV3 is produced as a propeptide and then cleaved to release the mature signaling molecule. Upon activation, the CLV1/2 complex blocks the activity of the PP2C proteins, POL and PLL1 via an unknown mechanism. When CLV3 is not present, POL and PLL1 are active and through unidentified signaling intermediates upregulate the homeodomain transcription factor, WUS, which is required for the maintenance and specification of the stem cells.
Initial studies of the pol and pll1 single mutants showed that these mutants have weak aberrant phenotypes and only partially rescue clv mutant phenotypes.3–5 These results strongly suggest that POL and PLL1 have overlapping functions. To follow up on this possibility, we recently analyzed pol pll1 double mutants and discovered they are seedling lethal because of a failure to develop the central vasculature or the root apical meristem.6 These defects were rescued by grafting pol pll1 shoots onto wild-type roots. The resulting plants displayed a wus mutant phenotype and further experiments demonstrated that the presence of either POL or PLL1 is required for the maintenance of WUS expression and stem cells.
While the pol pll1 shoot meristem defects can be attributed to disruption of the CLV pathway, the defects identified in the vasculature and the root apical meristem have no known connection to CLV pathway signaling. These phenotypes and the abnormal development of the pedicels and leaf vasculature observed in the POL and PLL1 single mutants and overexpressors,5 lead us to hypothesize that POL and PLL1 are shared machinery for a number of signaling pathways. This possibility is not surprising considering there are approximately 500 receptor-like kinases in Arabidopsis and only 76 members of the PP2C family.7,8 The concept of shared signaling machinery is common and can be seen with other cellular regulators, such as RAS and MAPK.9,10
Furthermore, misregulation of WUS expression alone does not account for all of the aberrant phenotypes seen in pol pll1 double mutants. Specifically, unlike the pol pll1 seedlings, wus mutants develop normal basal embryo tissues, which is consistent with the restriction of WUS expression to specific cell lineages in the apical half of the embryo during development.11,12 Thus, the pathways containing POL and PLL1 must regulate additional target genes. Obvious candidates are the 14 WUS-like (WOX) genes in Arabidopsis.13 Most notable is WOX5, whose mRNA accumulates in the upper lens-shaped cell that gives rise to the quiescent center, after the asymmetric division of the hypophyseal cell.13 This data, in addition to Medicago truncatula root expression studies, have led to the theory that WOX5 is required for the formation of root stem cell niches.14 One possible scenario is that POL and PLL1 are required for accumulation of WOX5 and/or other WOX family mRNAs in the upper lens-shaped cell. If this hypothesis is true, these mRNAs would not accumulate in the pol pll1 double mutants, resulting in disruption of quiescent center formation and causing the severe root defects observed.
On a final note, the restriction of WUS expression to the organizing center occurs through a series of asymmetric cell divisions where only one daughter cell continues to express WUS.12 Asymmetric cell divisions are also required for the generation of both the vascular tissue and the upper lens-shaped cell (mentioned above in connection with WOX5).15 Notably, the tissues resulting from all of these asymmetric cell divisions are defective in the pol pll1 double mutant. Perhaps, POL and PLL1 containing pathways establish or regulate these asymmetric cell divisions. Consistent with this idea are the directional cues inherent in CLV signaling. Specifically, the CLV3 signal travels basally to the organizing center to regulate WUS, while a yet undiscovered signal generated by WUS returns apically to maintain the stem cell population. Overall, further study is needed to determine how POL and PLL1 function in vivo to regulate development. As a first step, it is necessary to obtain a better understanding of what changes occur during the embryonic development of pol pll1 double mutants to result in the severe defects observed.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/3863
References
- 1.Doerner P. Plant meristems: A merry-go-round of signals. Curr Biol. 2003;13:R368–R374. doi: 10.1016/s0960-9822(03)00280-x. [DOI] [PubMed] [Google Scholar]
- 2.Sharma VK, Carles C, Fletcher JC. Maintenance of stem cell populations in plants. Proc Natl Acad Sci USA. 2003;100:11823–11829. doi: 10.1073/pnas.1834206100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu LP, Simon EJ, Trotochaud AE, Clark SE. POLTERGEIST functions to regulate meristem development downstream of the CLAVATA loci. Development. 2000;127:1661–1670. doi: 10.1242/dev.127.8.1661. [DOI] [PubMed] [Google Scholar]
- 4.Yu LP, Miller AK, Clark SE. POLTERGEIST encodes a protein phosphatase 2C that regulates CLAVATA pathways controlling stem cell identity at Arabidopsis shoot and flower meristems. Curr Biol. 2003;13:179–188. doi: 10.1016/s0960-9822(03)00042-3. [DOI] [PubMed] [Google Scholar]
- 5.Song SK, Clark SE. POL and related phosphatases are dosage-sensitive regulators of meristem and organ development in Arabidopsis. Dev Biol. 2005;285:272–284. doi: 10.1016/j.ydbio.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Song SK, Lee MM, Clark SE. POL and PLL1 phosphatases are CLAVATA1 signaling intermediates required for Arabidopsis shoot and floral stem cells. Development. 2006;133:4691–4698. doi: 10.1242/dev.02652. [DOI] [PubMed] [Google Scholar]
- 7.Shiu SH, Bleecker AB. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc Natl Acad Sci USA. 2001;98:10763–10768. doi: 10.1073/pnas.181141598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schweighofer A, Hirt H, Meskiene I. Plant PP2C phosphatases: Emerging functions in stress signaling. Trends Plant Sci. 2004;9:236–243. doi: 10.1016/j.tplants.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Campbell SL, Khosravi-Far R, Rossman KL, Clark GJ, Der CJ. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz MA, Madhani HD. Principles of MAP kinase signaling specificity in Saccharomyces cerevisiae. Annu Rev Genet. 2004;38:725–748. doi: 10.1146/annurev.genet.39.073003.112634. [DOI] [PubMed] [Google Scholar]
- 11.Laux T, Mayer KF, Berger J, Jurgens G. The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development. 1996;122:87–96. doi: 10.1242/dev.122.1.87. [DOI] [PubMed] [Google Scholar]
- 12.Mayer KF, Schoof H, Haecker A, Lenhard M, Jurgens G, Laux T. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell. 1998;95:805–815. doi: 10.1016/s0092-8674(00)81703-1. [DOI] [PubMed] [Google Scholar]
- 13.Haecker A, Gross-Hardt R, Geiges B, Sarkar A, Breuninger H, Herrmann M, Laux T. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development. 2004;131:657–668. doi: 10.1242/dev.00963. [DOI] [PubMed] [Google Scholar]
- 14.Imin N, Nizamidin M, Wu T, Rolfe BG. Factors involved in root formation in Medicago truncatula. J Exp Bot. 2006 doi: 10.1093/jxb/erl224. In Press. [DOI] [PubMed] [Google Scholar]
- 15.Scheres B, Benfey PN. Asymmetric cell division in plants. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:505–537. doi: 10.1146/annurev.arplant.50.1.505. [DOI] [PubMed] [Google Scholar]