Abstract
There are accumulating reports that polyamines are involved in abiotic stress response. However, the role played by the polyamines is not fully elucidated. In the present studies, we assessed whether spermine among the polyamines plays a certain role against high salt and drought stresses using an Arabidopsis (acl5/spms) mutant plant that does not produce spermine, and found that it was hypersensitive to those stresses. In each case the hypersensitive phenotype was mitigated by application of exogenous spermine. The spermine-deficient mutant plants also showed a phenotype resembling Ca2+-deficiency. The NaCl-hypersensitivity and Ca2+-deficiency of acl5/spms double-knockout mutant resembled the phenotypes displayed by the AtGluR2- and CAX1-overexpressing transgenic plants. The two latter genes encode a glutamate receptor-type, Ca2+-ion influx channel at cytoplasmic membrane and a vacuolar Ca2+/H+ antiporter, respectively. The data suggest that regulated expression of the Ca2+-pathway members is critical to adapt to those stresses, and that spermine plays a certain role to control the stress-induced Ca2+ dynamics. Incorporating the current information from the literature, especially regarding action of polyamines on various ion channels, we present models describing a defensive role of spermine in high salt and drought stresses in Arabidopsis.
Key Words: Arabidopsis thaliana, calcium ion, drought stress, high salt stress, ion channels, polyamine, spermine
Salinity and water availability in soils are limiting factors for plant growth and productivity. Once plants encounter those stresses, they respond adaptively at transcriptional and translational levels.1 In both cases, one countermeasure employed by plants is to modulate polyamine biosynthesis, however, its significance has not been elucidated well.2 In our studies, a spermine-deficient mutant3 was used to assess the role of spermine during high salt and drought stresses.
The spermine-deficient mutant showed hypersensitivity to high salt and this phenotype was mitigated by exogenously applied spermine, but not by spermidine and putrescine. NaCl hypersensitivity of the mutant was also attenuated by treatment with Ca2+ channel blockers. More interestingly, the mutant displayed a phenotype resembling that of Ca2+ deficiency. Both, the NaCl-hypersensitivity and Ca2+-deficiency resembled the phenotypes displayed by the AtGluR2- and CAX1-overexpressing transgenic plants.4,5 The former gene encodes a glutamate receptor-type, Ca2+-ion channel located in plasma membrane and the latter encodes a vacuolar Ca2+/H+ antiporter. The data indicate that coordinated expression of the Ca2+-pathway members is essential to adapt to high salt stress, and that spermine plays a certain role to control the stress-induced Ca2+ dynamics.
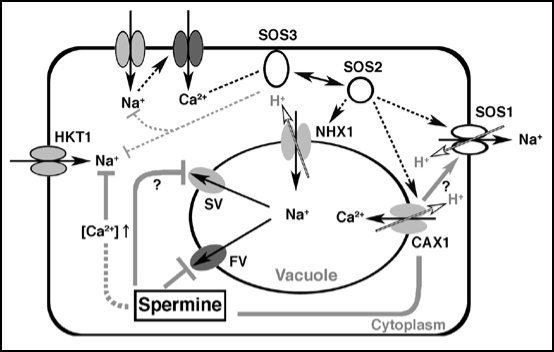
It is known that the balance of Ca2+ and Na+ ions is critical to adapt to high salt stress in plants.6 Ca2+ acts not only as a second messenger but also inhibits Na+ entry into cells.7–9 In Arabidopsis, initial entry of Na+ ions triggers a rapid but transient increase of cytosolic Ca2+ and this change is sensed by SOS3, a Ca2+ binding protein with sequence similarity to the regulatory subunit of calcineurin (Fig. 1). The conformational change of SOS3 induced by Ca2+ leads to interaction with SOS2, a protein kinase of the SNF1-related kinase family. This physical interaction activates SOS2 phosphorylation of SOS1, a plasma membrane Na+/H+ antiporter, resulting in efflux of excess Na+ ions. This series of responses is designated ‘SOS-signaling pathway’.10 Furthermore, the SOS2/SOS3 complex inhibits the activity of HKT1, a low affinity Na+ transporter, to prevent Na+ entry to cytoplasm. SOS2 has a multiple function. In addition to participation in the SOS-signaling pathway it is an activator of NHX, encoding a vacuolar Na+/H+ exchanger, to sequestrate excess Na+ ions to vacuoles. SOS2 interacts with and activates CAX1,8 which shows a strong link between Ca2+ and Na+ homeostatis in plants. In addition, overexpression of the CAX1 gene causes salt sensitivity, suggesting the importance of the coordinated regulation of Na+ and Ca2+ transporters during high salt stress.
Figure 1.
A hypothetical model of the defensive role of spermine in high NaCl-stressed Arabidopsis.
Brüggemann et al.11 showed that cytoplasmic polyamines block a fast-activating vacuolar channel (FV) from barley. Among polyamines, spermine was the most potent blocker. Similarly, FV and slow-activating vacuolar (SV) channels from red beet taproots were inhibited by polyamines.12,13 Again, the potency of this action was of the same order: spermine > spermidine > putrescine. The sensitivity of the FV to spermine and spermidine was much higher compared to the one of SV. The FV is a cation channel for monovalent ions, preferentially K+.14 The SV is a cation channel for monovalent- and divalent ions with lower specificity.15,16
Collectively we integrate a role of spermine against high salt stress (Fig. 1). Firstly, spermine blocks the Na+ leakage from monovalent cation-permeable FV channels.12 It can not be ruled out that spermine also blocks SV channels. However, the minimal dose of spermine for action on FV is a few µM whereas for SV it is a few hundred µM in red beet vacuoles. Secondly, spermine may control Ca2+ allocation through regulating Ca2+-permeable channels including CAXs. In NaCl-treated spermine-deficient mutant plants, the expression of CAX1 and CAX3 genes was upregulated to ca. 1.9- and 5.5-fold, respectively. A single mutant of CAX1 and CAX3 displayed no obvious phenotype, while the double mutant (cax1/cax3) exhibited a severe reduction of growth and abnormal ion profiles.17 Lastly, change of Ca2+ compartmentation induced by spermine is a strong possibility to prevent Na+ entry through HKT1-type channels and enhance SOS1 activity.
The spermine-deficient mutant plant was also hypersensitive to drought. This phenotype was recovered by the pretreatment with spermine. When exposed to drought condition, the mutant plant lost more water than the wild type plant because the mutant plant did not close stomata quickly enough. This phenomenon may again be explained by the impairment in Ca2+ homeostasis. Changes of free Ca2+ in the cytoplasm of guard cells are involved in stomatal movement.18,19 Spermine produced by drought stress may modulate Ca2+-permeable channels, resulting in the increase of cytoplasmic Ca2+ concentration to trigger stomatal closure.20 Reported that polyamines also induce closure of stomata through inhibiting K+ channel.21
Our studies demonstrated an impact of spermine action during high salt and drought stresses. To gain a deeper understanding of spermine's role during abiotic stresses, we have to study the action of polyamines, especially spermine on ion channels.
Acknowledgements
We thank to Drs. Anthony J. Michael, Taku Takahashi, and Gerald Schönknecht for their sincere discussion. We also thank to Dr. Matthew R. Shenton for critically reading the manuscript. This work is partially supported by ‘The Salt Science Foundation’.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/3866
References
- 1.Zhu JK. Salt and drought stress signal transduction in plant. Annu Rev Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouchereau A, Aziz A, Larher F, Martin-Tanguy J. Polyamines and environmental challenges: Recent development. Plant Sci. 1999;140:103–125. [Google Scholar]
- 3.Imai A, Akiyama T, Kato T, Sato S, Tabata S, Yamamoto KT, Takahashi T. Spermine is not essential for survival of Arabidopsis. FEBS Lett. 2004;556:148–152. doi: 10.1016/s0014-5793(03)01395-4. [DOI] [PubMed] [Google Scholar]
- 4.Kim SA, Kwak JM, Jae SK, Wang MH, Nam HG. Overexpression of the AtGluR2 gene encoding an Arabidopsis homolog of mammalian glutamate receptors impairs calcium utilization and sensitivity to ionic stress in transgenic plants. Plant Cell Physiol. 2001;42:74–84. doi: 10.1093/pcp/pce008. [DOI] [PubMed] [Google Scholar]
- 5.Hirschi KD. Expression of Arabidopsis CAX1 in tobacco: Altered calcium homeostasis and increased stress sensitivity. Plant Cell. 1999;11:2113–2122. doi: 10.1105/tpc.11.11.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niu X, Bressan RA, Hasegawa PM, Pardo JM. Ion homeostasis in NaCl stress environments. Plant Physiol. 1995;109:735–742. doi: 10.1104/pp.109.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knight H, Trewavas AJ, Knight MR. Calcium signaling in Arabidopsis thaliana responding to drought and salinity. Plant J. 1997;12:1067–1078. doi: 10.1046/j.1365-313x.1997.12051067.x. [DOI] [PubMed] [Google Scholar]
- 8.Sanders D, Pelloux J, Brownlee C, Harper JF. Calcium at the crossroads of signaling. Plant Cell. 2002;14:S401–S417. doi: 10.1105/tpc.002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng NH, Pittman JK, Zhu JK, Hirschi KD. The protein kinase SOS2 activates the Arabidopsis H+/Ca2+ antiporter CAX1 to integrate calcium transport and salt tolerance. J Biol Chem. 2004;279:2922–2926. doi: 10.1074/jbc.M309084200. [DOI] [PubMed] [Google Scholar]
- 10.Zhu JK. Plant salt tolerance. Trends Plant Sci. 2001;6:66–71. doi: 10.1016/s1360-1385(00)01838-0. [DOI] [PubMed] [Google Scholar]
- 11.Brüggemann L, Pottosin I, Schönknecht G. Cytoplasmic polyamines block the fast-activating vacuolar cation channel. Plant J. 1998;16:101–105. [Google Scholar]
- 12.Dobrovinskaya OR, Muñiz J, Pottosin II. Inhibition of vacuolar ion channels by polyamines. J Membr Biol. 1999;167:127–140. doi: 10.1007/s002329900477. [DOI] [PubMed] [Google Scholar]
- 13.Dobrovinskaya OR, Muñiz J, Pottosin II. Asymmetric block of the plant vacuolar Ca2+-permeable channel by organic cations. Eur Biophys J. 1999;28:552–563. doi: 10.1007/s002490050237. [DOI] [PubMed] [Google Scholar]
- 14.Brüggemann L, Pottosin I, Schönknecht G. Selectivity of the fast activating vacuolar cation channel. J Exp Bot. 1999;50:873–876. [Google Scholar]
- 15.Barkla BJ, Pantoja O. Physiology of ion transport across the tonoplast of higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:159–184. doi: 10.1146/annurev.arplant.47.1.159. [DOI] [PubMed] [Google Scholar]
- 16.Ward JM, Schroeder JI. Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell. 1994;6:669–683. doi: 10.1105/tpc.6.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng NH, Pittman JK, Shigaki T, Lachmansingh J, LeClere S, Lahner B, Salt DE, Hirschi KD. Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol. 2005;138:2048–2060. doi: 10.1104/pp.105.061218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen GJ, Sanders D. Control of ionic currents guard cell vacuoles by cytosolic and luminal calcium. Plant J. 1996;10:1055–1069. doi: 10.1046/j.1365-313x.1996.10061055.x. [DOI] [PubMed] [Google Scholar]
- 19.Peiter E, Maathuis FJM, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature. 2005;434:404–408. doi: 10.1038/nature03381. [DOI] [PubMed] [Google Scholar]
- 20.Maathuis FJM, Ichida AM, Sanders D, Schroeder JI. Roles of higher plant K+ channels. Plant Physiol. 1997;114:1141–1149. doi: 10.1104/pp.114.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu K, Fu H, Bei Q, Luan S. Inward potassium channel in guard cells as a target for polyamine regulation of stomatal movements. Plant Physiol. 2000;124:1315–1326. doi: 10.1104/pp.124.3.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]