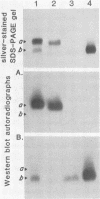
Abstract
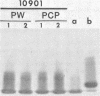
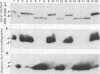
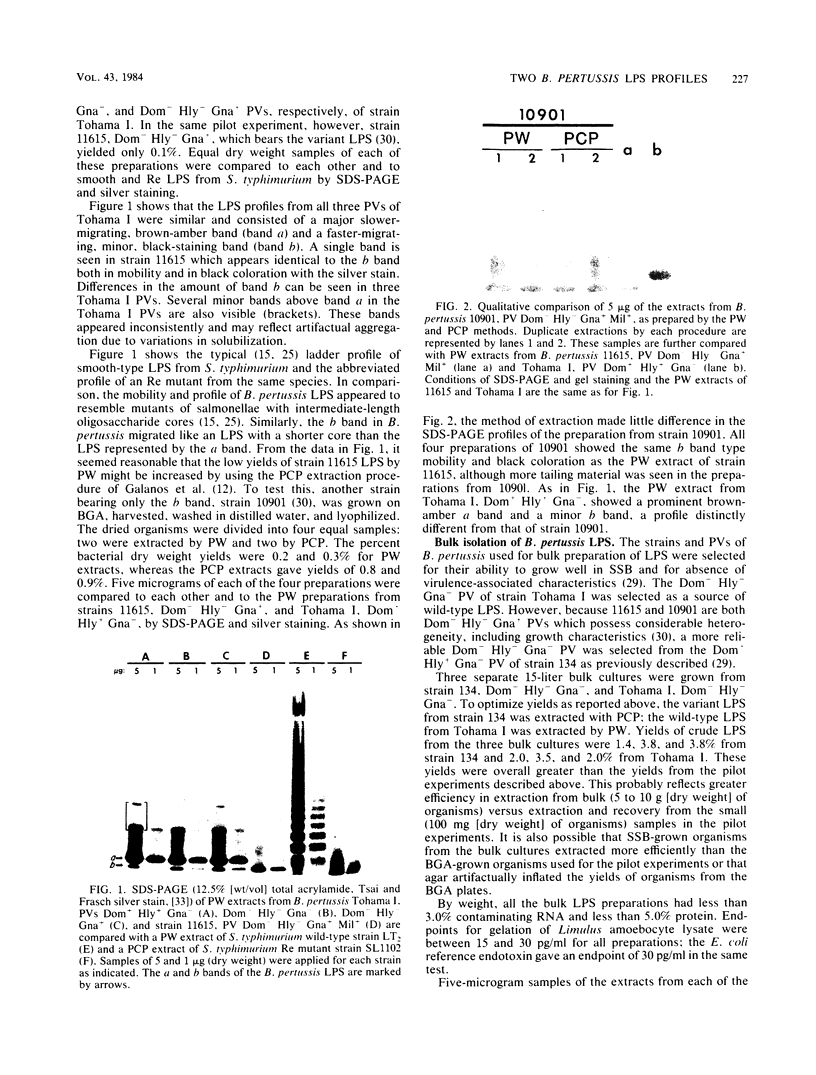
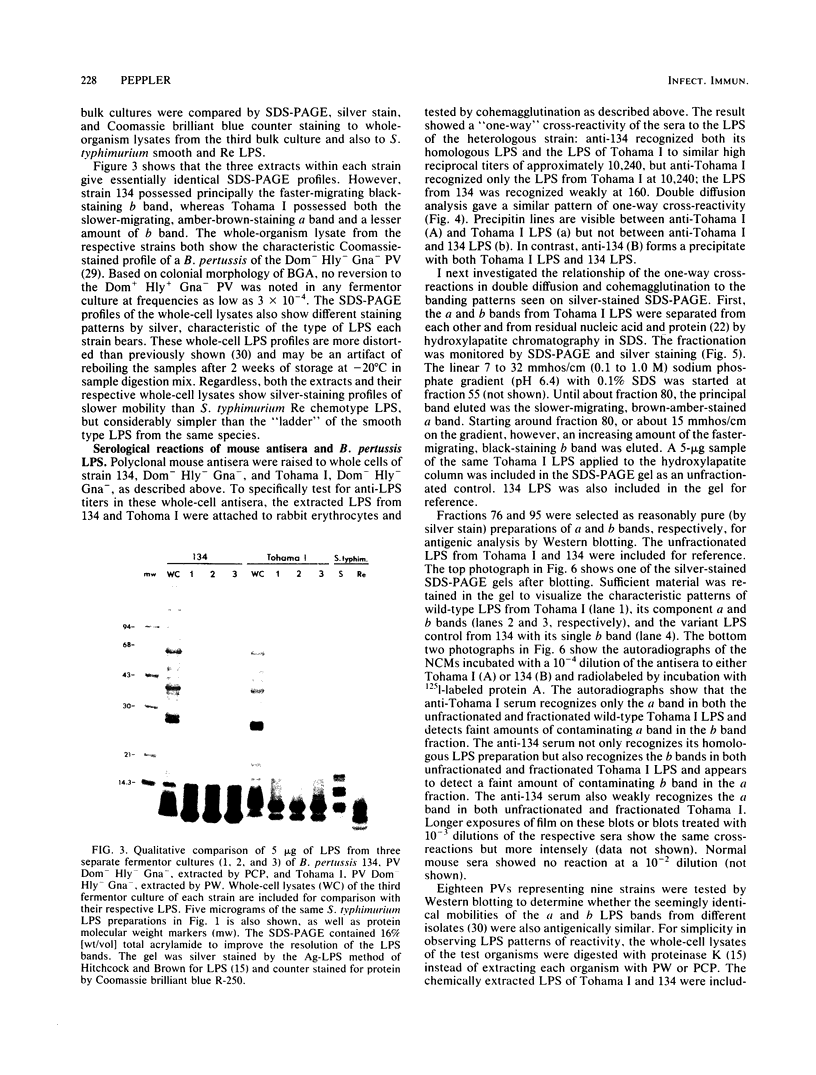
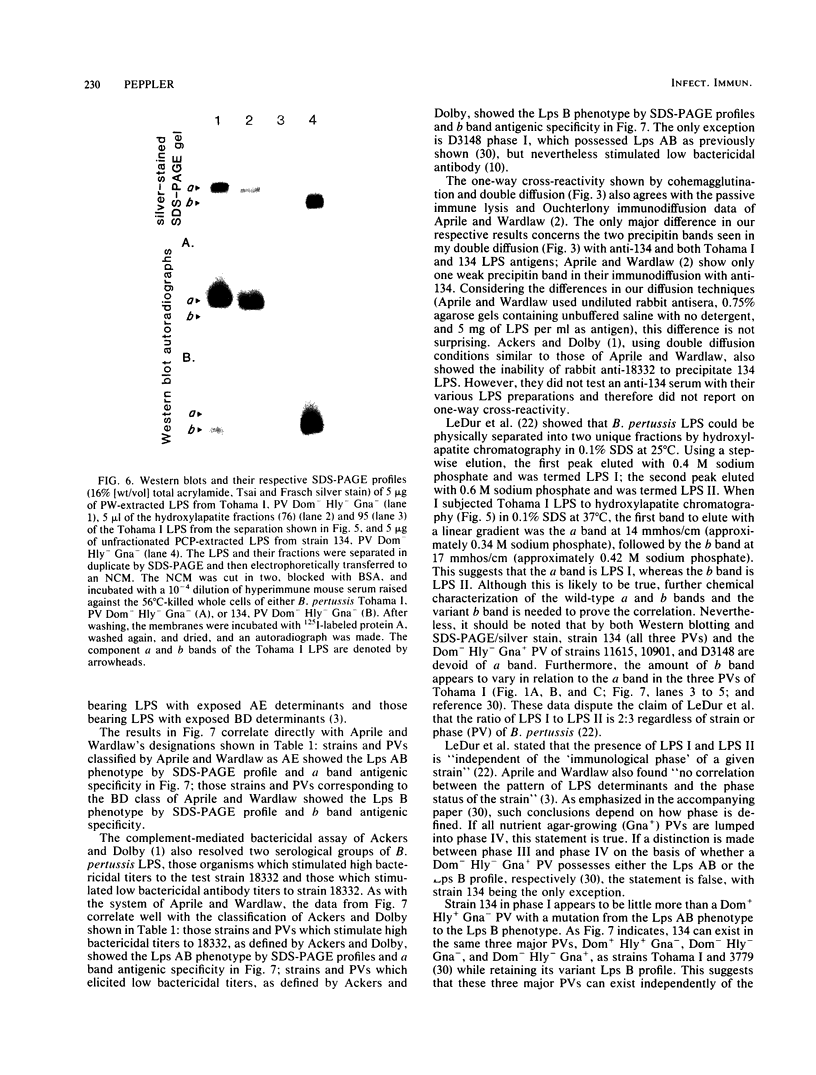
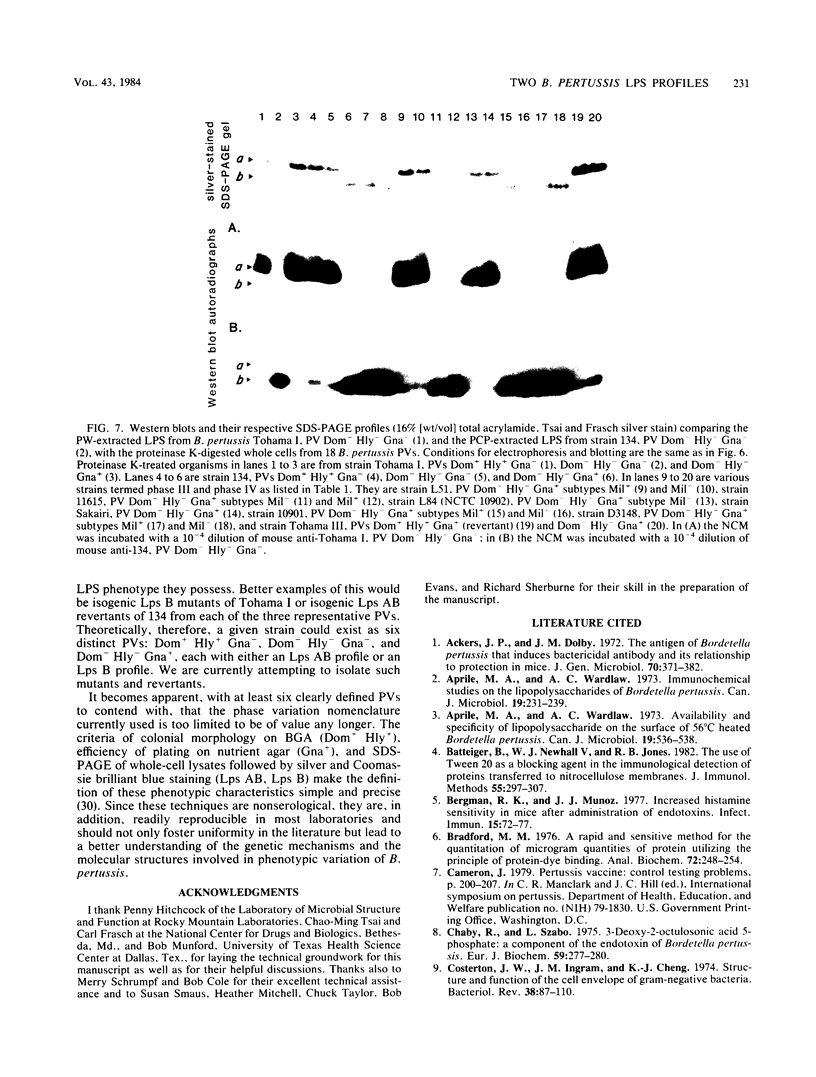
The lipopolysaccharide (LPS) from nine strains representing 18 phenotype variants of Bordetella pertussis could be grouped into one of two distinct profiles by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and silver staining. One group, representing the wild-type LPS profile of B. pertussis, consisted of two silver-staining bands: a dominant brown-amber a band and a faster-migrating, minor, black-staining b band. The second group, representing a variant LPS profile, consisted of a single black-staining band of similar mobility to the b band in the wild-type profile. By electrophoretic transfer (Western) blot analysis, mouse antiserum raised against whole cells of Tohama I (prototype wild-type LPS strain) recognized only the a band from all strains/phenotypes possessing the wild-type LPS profile. In contrast, mouse antiserum raised against whole cells of 134 (prototype variant LPS strain) recognized all b bands, regardless of strain/phenotype, and could be shown to cross-react weakly with the a band from Tohama I. These results and results from cohemagglutination and immunodiffusion analyses support the classification of B. pertussis into one of two physiologically and serologically distinct LPS phenotypes: Lps AB for the wild-type profile and Lps B for the variant profile. The relationship of LPS type and phenotypic, or "phase," variation is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackers J. P., Dolby J. M. The antigen of Bordetella pertussis that induces bactericidal antibody and its relationship to protection of mice. J Gen Microbiol. 1972 Apr;70(2):371–382. doi: 10.1099/00221287-70-2-371. [DOI] [PubMed] [Google Scholar]
- Aprile M. A., Wardlaw A. C. Availability and specificity of lipopolysaccharide on the surface of 56 degrees C heated Bordetella pertussis. Can J Microbiol. 1973 Apr;19(4):536–538. doi: 10.1139/m73-087. [DOI] [PubMed] [Google Scholar]
- Aprile M. A., Wardlaw A. C. Immunochemical studies on the lipopolysaccharides of Bordetella pertussis. Can J Microbiol. 1973 Feb;19(2):231–239. doi: 10.1139/m73-035. [DOI] [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Bergman R. K., Munoz J. J. Increased histamine sensitivity in mice after administration of endotoxins. Infect Immun. 1977 Jan;15(1):72–77. doi: 10.1128/iai.15.1.72-77.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chaby R., Szabó L. 3-Deoxy-2-octulosonic acid 5-phosphate: a component of the endotoxin of Bordetella pertussis. Eur J Biochem. 1975 Nov 1;59(1):277–280. doi: 10.1111/j.1432-1033.1975.tb02452.x. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolby J. M., Ackers J. P. Taxonomic distribution of the antigen eliciting bactericidal antibody for Bordetella pertussis. J Gen Microbiol. 1975 Apr;87(2):239–244. doi: 10.1099/00221287-87-2-239. [DOI] [PubMed] [Google Scholar]
- Finger H., Heymer B., Hof H., Rietschel E., Schleifer K. H. Uber Struktur und biologische Aktivität von Bordetella pertussis-Endotoxin. Zentralbl Bakteriol Orig A. 1976 Aug;235(1-3):56–64. [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Griffith A. H. Reactions after pertussis vaccine: a manufacturer's experiences and difficulties since 1964. Br Med J. 1978 Apr 1;1(6116):809–815. doi: 10.1136/bmj.1.6116.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASUGA T., NAKASE Y., UKISHIMA K., TAKATSU K. Studies on Haemophilis pertussis. III. Some properties of each phase of H. pertussis. Kitasato Arch Exp Med. 1954 Sep;27(3):37–47. [PubMed] [Google Scholar]
- KASUGA T., NAKASE Y., UKISHIMA K., TAKATSU K. Studies on Haemophilus pertussis. I. Antigen structure of H. pertussis and its phases. Kitasato Arch Exp Med. 1953 Nov;26(2-3):121–133. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kurokawa M., Ishida S., Asakawa S., Iwasa S. Attempts at analysis of toxicity of pertussis vaccine. III. Effects of endotoxin on leukocytosis in mice due to lymphocytosis-promoting factor and reference preparations for determination of lymphocytosis-promoting factor. Jpn J Med Sci Biol. 1978 Apr;31(2):91–103. doi: 10.7883/yoken1952.31.91. [DOI] [PubMed] [Google Scholar]
- Le Dur A., Caroff M., Chaby R., Szabó L. A novel type of endotoxin structure present in Bordetella pertussis. Isolation of two different polysaccharides bound to lipid A. Eur J Biochem. 1978 Mar 15;84(2):579–589. doi: 10.1111/j.1432-1033.1978.tb12201.x. [DOI] [PubMed] [Google Scholar]
- Le Dur A., Chaby R., Szabó L. Isolation of two protein-free and chemically different lipopolysaccharides from Bordetella pertussis phenol-extracted endotoxin. J Bacteriol. 1980 Jul;143(1):78–88. doi: 10.1128/jb.143.1.78-88.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACLENNAN A. P. Specific lipopolysaccharides of Bordetella. Biochem J. 1960 Feb;74:398–409. doi: 10.1042/bj0740398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe W. R. Immunization with R mutants of S. Minnesota. I. Protection against challenge with heterologous gram-negative bacilli. J Immunol. 1972 Mar;108(3):601–610. [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Rick P. D. Size heterogeneity of Salmonella typhimurium lipopolysaccharides in outer membranes and culture supernatant membrane fragments. J Bacteriol. 1980 Nov;144(2):630–640. doi: 10.1128/jb.144.2.630-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NETER E., BERTRAM L. F., ZAK D. A., MURDOCK M. R., ARBESMAN C. E. Studies on hemagglutination and hemolysis by escherichia coli antisera. J Exp Med. 1952 Jul;96(1):1–15. doi: 10.1084/jem.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase Y., Tateishi M., Sekiya K., Kasuga T. Chemical and biological properties of the purified O antigen of Bordetella pertussis. Jpn J Microbiol. 1970 Jan;14(1):1–8. doi: 10.1111/j.1348-0421.1970.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Peppler M. S. Isolation and characterization of isogenic pairs of domed hemolytic and flat nonhemolytic colony types of Bordetella pertussis. Infect Immun. 1982 Mar;35(3):840–851. doi: 10.1128/iai.35.3.840-851.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppler M. S., Schrumpf M. E. Isolation and characterization of Bordetella pertussis phenotype variants capable of growing on nutrient agar: comparison with phases III and IV. Infect Immun. 1984 Jan;43(1):217–223. doi: 10.1128/iai.43.1.217-223.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel E. T., Schade U., Jensen M., Wollenweber H. W., Lüderitz O., Greisman S. G. Bacterial endotoxins: chemical structure, biological activity and role in septicaemia. Scand J Infect Dis Suppl. 1982;31:8–21. [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]