Abstract
Stomata regulate gas exchange and their closure in response to pathogens may, in some cases, contribute to resistance. However, in the cereal mildew and rust systems, stomatal closure follows establishment of compatible infections. In incompatible systems, expression of major (R) gene controlled hypersensitive responses (HR), causes drastic, permanent stomatal dysfunction: stomata become locked open following powdery mildew attack and locked shut following rust attack. Thus, stomatal locking can be a hitherto unsuspected negative consequence of R gene resistance that carries a physiological cost affecting plant performance.
Key Words: stomata, rust, mildew, hypersensitive response, stomatal lock-up
Stomata: Guarded Gates to Pathogens?
Stomata balance CO2 uptake with transpiration1 in response to physiological and environmental cues and they also respond to biotic stress including pathogen attack. Recently Mellotto et al.2 showed that bacterial pathogen-associated molecular patterns trigger rapid stomatal closure, and argued that this represents an innate mechanism of resistance against pathogens that enter via stomatal pores. Our published study showed that rapid stomatal closure is also a pre-penetration response of barley to the powdery mildew fungus, Blumeria graminis f. sp. hordei (Bgh),3 but as Bgh does not enter via stomata, the effect, if any, of this closure on the pathogen is not direct.
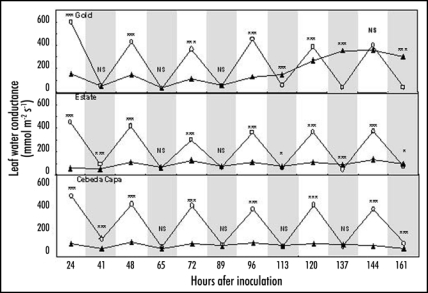
Bgh is an obligate biotroph with an ectophytic habit; it penetrates only into epidermal cells to form feeding structures (haustoria) that supply the surface mycelia and reproductive structures. Our observations of compatible interactions confirmed earlier studies4 showing that stomata in the vicinity of established colonies lost the ability to open in response to light, thus reducing leaf water conductance (gl). In line with earlier studies of bean rust,5 we recently found a very similar effect of infection by brown rust fungi in susceptible barley (cv. Gold, Fig. 1) and wheat (cv. Thatcher, Fig. 2). Although these rusts are also obligate biotrophs, they enter leaves via stomata and feed largely on mesophyll cells before rupturing the epidermis at the onset of sporulation. Thus, our data (Figs. 1 and 2) show low gl, indicating failure of stomatal opening in light, until the epidermis was ruptured some time after infection. While stomatal closure clearly did not prevent disease infection by the rusts, like Bgh, has obvious and serious implications for physiological activity mediated by stomatal movement. Interestingly, one consequence of this stomatal closure must be reduced photosynthesis thereby reducing the potential supply of assimilates available to the pathogens. Together with the premature leaf senescence associated with infection, this must limit colony growth, sporulation capacity and longevity of infections and thereby limit spread of disease. This would be a rather perverse form of ‘resistance’ that is a consequence of susceptibility.
Figure 1.
Leaf water conductance in healthy (○) and P. hordei, race octal BRS 273, isolate 03-23 attacked (▴) leaves of barley cvs Gold (susceptible), Estate (Infection Tipe (IT) = 0c; R gene Rph 3) and Cebeda Capa (IT = 0n; R gene Rph 7) in successive light (unshaded) and dark periods (grey shaded) after inoculation. Comparing within sample times: NS, no significant difference; *p < 0.05; ***p < 0.001.
Figure 2.
Leaf water conductance in healthy (○) and P. triticina, isolate WBRS-04-02, attacked (▴) leaves of wheat lines Thatcher (susceptible), Lr20 (IT = 0c,n) and Lr24 (IT = mainly 0c but with a few small pustules) in successive light (unshaded) and dark periods (grey shaded) after inoculation. Comparing within sample times: NS, no significant difference; *p < 0.05; **p < 0.01; ***p < 0.001.
Stomata in Lock-up: Paying the Penalty for the Hypersensitive Response?
Major (R) genes conveying race-specific disease resistance associated with a hypersensitive response (HR) are commonly deployed to control crop pathogens. Our previous3 and unpublished studies of Bgh interactions indicate that expression of HR in the barley powdery mildew system has a common consequence leading to stomata becoming locked-open and unable to close in response to darkness, extreme drought or application of abscisic acid, even though plants appeared disease free. To test further the generality of this effect, we examined the consequences of R gene resistance to brown rust in barley and wheat. The reaction of barley cv. Estate (R gene Rph 3) is characterised by a chlorotic response, and of cv. Cebeda Capa (R gene Rph 7) by a necrotic response while the wheat line carrying the R gene Lr20 gives mixed chlorotic and necrotic responses (ERL Jones, pers. comm.); no sporulation occurs in any of these cases. The wheat line with R gene Lr24 shows some chlorosis and allows a very few small rust pustules to develop. As in the susceptible (cv. Gold), both resistant barley lines showed substantial rust attack-induced suppression of gl in the light, indicating failure of stomatal opening, but little effect on dark gl (Fig. 1). The wheat genotype Lr20 showed a similar effect though here gl in the light period was suppressed more than in Thatcher (Fig. 2). In all these resistant lines, light gl remained low for the entire duration of experiments. Thus, despite lack of visible disease, expression of resistance led to a permanent, severe impairment of stomatal opening. By contrast, in the wheat Lr24 reduction in light gl was evident but remained relatively small (Fig. 2).
Thus, whilst R gene resistance to powdery mildew causes stomatal lock-open, in the rust systems the opposite is true. A possible explanation for lock-open associated with powdery mildew HR is breakdown of turgor balance between epidermal and guard cells. Turgid epidermal cells provide back-pressure on neighbouring guard cells that reduces stomatal aperture by c. 50%.1,6,7 and this would be lost as a result of epidermal HR. By contrast, since HR to rust attack would mainly affect the mesophyll, epidermal/stomatal turgor relations may not be disrupted. Nevertheless, differences might also arise from impact of their epiphytic and endophytic habits on plant signalling systems. However, recent work reported stomatal lock-open in grapevine following Plasmopara viticola infection,8 an endophytic biotrophic oomycete that infects mesophyll tissue. Clearly, future work must unravel the mechanism(s) leading to stomatal dysfunction in different pathosystems.
Though we do not understand the mechanisms causing stomatal dysfunction, they must contribute substantially to the ‘cost of resistance’, a phenomenon that has been recognised but evaded good explanation.9,10 Stomatal dysfunction will interfere with photosynthesis, respiration, transpiration and ability to cope with abiotic stresses. Such stresses may be come even more important in the face of environmental change and the requirement for increased water use efficiency. For sustainable production systems where powdery mildew is a threat, a good alternative is offered by broad spectrum, papilla-based resistance, which leads to only transient impairment of stomatal opening.3 In rust systems, genes such as Lr24 may prove useful. More extensive physiological and field studies are required to explore the implications of HR for costs of resistance, to identify strategies to minimise the impact of disease and to quantify the trade off between disease resistance and physiological dysfunction.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4153
References
- 1.Roelfsema MRG, Hedrich R. In the light of stomatal opening: New insights into ‘the Watergate’. New Phytol. 2005;167:665–691. doi: 10.1111/j.1469-8137.2005.01460.x. [DOI] [PubMed] [Google Scholar]
- 2.Melotto M, Underwood W, Koczan J, Nomura K, He SY. Plant stomata function in innate immunity against bacterial invasion. Cell. 2006;126:969–980. doi: 10.1016/j.cell.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 3.Prats E, Gay AP, Mur LAJ, Thomas BJ, Carver TLW. Stomatal lock-open, a consequence of epidermal cell death, follows transient suppression of stomatal opening in barley attacked by Blumeria graminis. J Exp Bot. 2006;57:2211–2226. doi: 10.1093/jxb/erj186. [DOI] [PubMed] [Google Scholar]
- 4.Ayres PG, Zadoks JC. Combined effects of powdery mildew disease and soil water level on the water relations and growth of barley. Physiol Plant Pathol. 1979;14:347–361. [Google Scholar]
- 5.Ayres PG. Stomatal Physiology. In: Jarvis PG, Mansfield TA, editors. Soc Exp Biol Seminar. Cambridge University Press: 1981. pp. 205–222. (Series 8). [Google Scholar]
- 6.Felle HH, Hanstein S, Steinmeyer R, Hedrich R. Dynamics of ionic activities in the apoplast of the sub-stomatal cavity of intact Vicia faba leaves during stomatal closure evoked by ABA and darkness. Plant J. 2000;24:297–304. doi: 10.1046/j.1365-313x.2000.00878.x. [DOI] [PubMed] [Google Scholar]
- 7.Klein M, Cheng G, Chung M, Tallman G. Effects of turgor potentials of epidermal cells neighbouring guard cells on stomatal opening in detached leaf epidermis and intact leaflets of Vicia faba L. (faba bean) Plant Cell Environ. 1996;19:1399–1407. [Google Scholar]
- 8.Allègre M, Daire X, Héloir MC, Trouvelot S, Mercier L, Adrian M, Pugin A. Stomatal deregulation in Plasmopara viticola infected grapevine leaves. New Phytol. 2007;173:832–840. doi: 10.1111/j.1469-8137.2006.01959.x. [DOI] [PubMed] [Google Scholar]
- 9.Brown JKM. Yield penalties of disease resistance in crops. Curr Opin Plant Biol. 2002;5:339–344. doi: 10.1016/s1369-5266(02)00270-4. [DOI] [PubMed] [Google Scholar]
- 10.Purrington CB. Costs of resistance. Curr Opin Plant Biol. 2000;3:305–308. doi: 10.1016/s1369-5266(00)00085-6. [DOI] [PubMed] [Google Scholar]