Abstract
AIR9 is an essential microtubule-associated protein from Arabidopsis. Sequence similarity searches indicate homologues of AIR9 in land plants and in excavate protists, including trypanosomatid parasites and Trichomonas. The AIR9-like protein from Trypanosoma brucei was recently detected in the proteome of the trypanosome flagellum, raising the possibility that trypanosomatid AIR9-like proteins also associate with microtubules. Because microtubule functions are essential to the viability of trypanosomatid parasites AIR9-like proteins may be exploited as drug targets without homology in humans. We further discuss the unexpected phylogeny of AIR9-like proteins from plants and protozoans.
Key Words: plant, cortical microtubules, preprophase band, A9 domain, flagellum, Excavata
Using a proteomic approach we recently determined that the protein encoded by the single-copy AIR9 (Auxin-Induced in Root Cultures 9) gene from Arabidopsis is a novel microtubule-associated protein (MAP). GFP-AIR9 associates with cortical microtubules of the plant interphase array and with the preprophase band (PPB). The plant-specific PPB is a transient microtubule array that marks the cortical division site and determines the plane of cytokinesis. The PPB disassembles at the onset of mitosis but leaves behind an unknown mark that during cytokinesis serves to attract the new cross-wall (the out-growing cell plate). GFP-AIR9 is not present at the PPB site during mitosis, but returns to this location when the cell plate inserts. Forming a torus, GFP-AIR9 then moves inward along the new cross-wall. By doing this, microtubule-associated AIR9 transiently behaves like a peripheral membrane protein. If cell plates are experimentally induced to insert at ectopic positions no AIR9 torus is formed. We conclude that AIR9 is capable of associating with a component of the enigmatic PPB memory.1
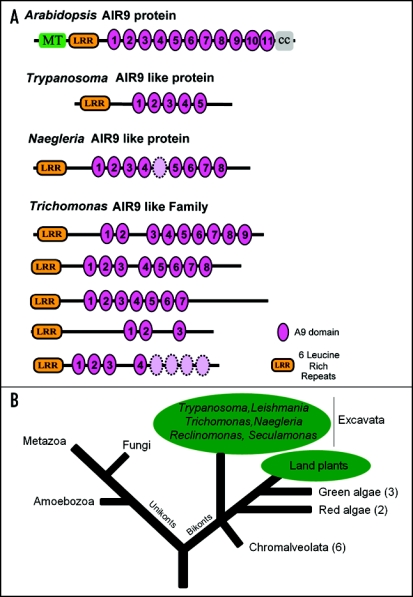
The 187 kDa Arabidopsis AIR9 protein consists of (seen from N- to C-terminus) a serine-rich microtubule-binding domain, a leucine-rich repeat (LRR) domain, a large region of eleven repeated A9 domains and a C-terminal region that is conserved among plant homologues (Fig. 1A). Our analyses revealed that A9 domains are likely to adopt g-immunoglobulin-like (IgG-like) folds. Using BLAST we detected AIR9 homologues in various land plants and in the genomes of the three sequenced trypanosomatid parasites.2 The trypanosomatid proteins contain the LRR domain and the region of repeated A9 domains found in the Arabidopsis prototype and were therefore termed AIR9-like proteins. PSI-BLAST searches3 indicate distantly related sequences with A9 domains in several prokaryotes (mainly proteobacteria) but these proteins are lacking the LRR domain.
Figure 1.
Domain structure of Arabidopsis AIR9 and protozoan AIR9-like proteins and phylogenetic position of AIR9-like proteins in the tree of life. (A) All AIR9-like proteins contain an LRR domain and a region of repeated A9 domains. Arabidopsis AIR9 additionally shows an N-terminal serine-rich basic region that confers microtubule binding (MT) and a C-terminal conserved region of unknown function (cc). The multicopy genome of Trichomonas harbours several genes potentially coding for AIR9-like proteins (shown in this order: gene1 EAY10472, gene2 EAY06037, gene3 EAX99086, gene4 EAY04681, gene5 EAX86457). (B) Phylogenetic tree showing key eukaryotic taxa. All organisms found to encode AIR9-like proteins are highlighted in green. Numbers in parenthesis indicate tested genomes devoid of AIR9-like proteins. This phylogenetic tree has been redrawn and is based on results published elsewhere.7,16,17
New genomic sequence data revealed the presence of AIR9-like proteins in the human parasite Trichomonas vaginalis4 (Fig. 1A). We intensified our searches for AIR9-like proteins in eukaryotes using EST datasets (http://tbestdb.bcm.umontreal.ca) and genome project BLAST servers (http://genome.jgi-psf.org) revealing a curious distribution of AIR9-like proteins in the eukaryotic tree of life (Fig. 1B). AIR9-like proteins are present in land plants (including the moss Physcomitrella) and in several protists of the excavate class (Trypanosomas, Leishmania, Trichomonas, Naegleria, Seculamonas, Reclinomonas). However, AIR9 is apparently missing in all other bikont organisms: it is missing in green algae (three sequenced genomes), in red algae (two sequenced genomes) and in all Chromalveolata sequenced to date (six genomes). As stated earlier,1 AIR9 is missing in all unikont organisms (metazoan, fungi, Amoebozoa) (Fig. 1B). This distribution of AIR9-like proteins is curious because land plants cannot be considered to be a sister group of Excavata.5,6 This may indicate a lateral gene transfer of AIR9-like genes between both groups. However, a different scenario may be based on independent losses of AIR9-like proteins in certain groups, i.e., in green and red algae, and possibly in Chromalveolata. It will be important to establish how widespread AIR9-like proteins are among organisms of the heterogenous Excavata. Unravelling the phylogenetic relationship between the groups Plantae, Chromalveolata and Excavata may explain this curious distribution of AIR9-like proteins in the tree of life.7–9
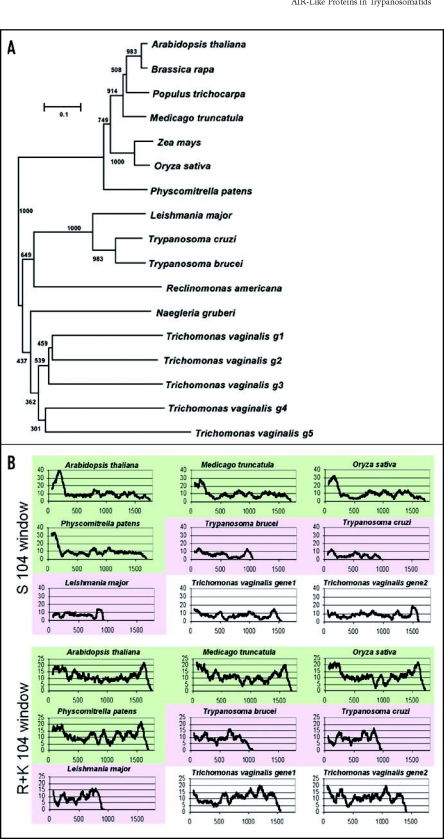
AIR9-like proteins are found in several important human parasites. Trypanosomatid parasites are the causal agent of African trypanosomiasis (sleeping sickness), Chagas disease and leishmaniasis. Other trypanosomatid species are pathogens of crops and live stock. Vaccines for humans are missing and effective drugs without side-effects are needed.2 Proteins restricted to plants and protists, as in the case of AIR9, could be exploited as drug targets against trypanosomatids -with no or little side-effects to humans.10,11 More plant-like traits were recently reported for Trypanosoma and Leishmania and assumed to stem from lateral gene-transfer events with an endosymbiosis-derived plastid organelle.12,13 It was speculated that trypanosomatid organisms once possessed an algal endosymbiont, but that this organelle was lost during evolution12,14 (for a different view, however, see Ref. 10). The phylogenetic comparison of AIR9-like proteins based on the LRR domain reveals two sub-classes of AIR9-like proteins resembling the groups land plants and excavate protists, respectively (Fig. 2A). The AIR9-like protein from the moss Physcomitrella patens tightly clusters with the sub-class from land plants. This is consistent with the last common ancestor of both AIR9 sub-classes being much older than the origin of land plants, excluding the possibility of a “very late” transfer of an AIR9-like gene between land plants and protists. Our findings on the phylogenetic distribution of AIR9 do not support nor reject the possibility of a green endosymbiont in early trypanosomatid organisms. They do, however, support the idea that new drug targets in trypanosomatid parasites could be discovered by analyzing drug targets from plants.
Figure 2.
Phylogenetic relationship of AIR9-like proteins and amino acid scans of plant and protozoan AIR9-like proteins. (A) Phylogenetic comparison based on the LRR domain reveals two subclasses of AIR9-like proteins corresponding to land plants and protozoans, respectively. (B) Amino-acid scans using a 104 amino-acid window. Note the serine-rich regions in AIR9 homologues from land plants all of which present N-terminal peaks above 20 serines in 104 amino acids. otherwise, the sequence similarity between these serine-rich regions is not statistically significant according to BLAST sequence similarity analyses [3]. Arginine plus lysine content in all plant homologues peak above 20 in 104 amino acids in N-terminal (and C-terminal) regions. The N-terminal basic region corresponds to the microtubule-binding domain found in Arabidopsis AIR9. N-terminal basic serine-rich extensions are not found in the AIR9-like proteins from trypanosomatid parasites. Some of the predicted AIR9-like proteins from Trichomonas peak with basic residues in the N-terminus. Green = plant proteins, pink = trypanosomatid proteins, white = Trichomonas proteins. Squences were obtained from protein databases, inferred from EST sequences, or derived from genomic sequences using homologous proteins as template. All sequences are available upon request.
Do the AIR9-like proteins from trypanosomatids and other excavates function in a similar way to AIR9 from plants, and are they MAPs? In a remarkable study Broadhead et al15 isolated the flagellum from blood-stream Trypanosoma brucei and identified its proteome by HPLC-assisted mass-spectrometry. RNA-interference with selected proteins of the flagellar proteome established that flagellar motility is essential for viability of the blood-stream Trypanosoma.15 The flagellum of trypanosomatids consists of the 9+2 axoneme and an associated structure, the paraflagellar rod. Interestingly, the trypanosomatid AIR9-like protein (Tb11.01.8770) is among the 331 proteins of the flagellum proteome (see supplemental data by Broadhead et al Ref. 15). This raises the possibility that the AIR9-like protein from Trypanosoma binds microtubules. In Arabidopsis AIR9, the N-terminal basic serine-rich extension functions in microtubule binding.1 The basic residues of this extension may confer binding to the acidic surface of tubulins. However, in linear alignments the microtubule binding site of Arabidopsis AIR9 appears to be only weakly conserved among land plants and no similarity to the AIR9-like proteins from protozoans is found (data not shown). We therefore scanned AIR9-like proteins from plants, trypanosomatid parasites and Trichomonas for serine-rich and basic regions. Using a 104 amino-acid window we found that all investigated plant proteins show a serine-rich and basic N-terminal extension. The AIR9-like proteins from trypanosomatid parasites do not show N-terminal serine-rich regions and the content of basic residues in these regions is far less pronounced when compared to the plant homologues. Trichomonas encodes at least five AIR9-like proteins, probably as a result of genome amplification events observed in this parasite.4 Interestingly, some of the putative Trichomonas AIR9-like proteins contain N-terminal basic regions (Fig. 2B). However, this is not mirrored by an elevated serine content. Taken together, these results show that all AIR9-like proteins from land plants exhibit N-terminal basic serine-rich extensions and therefore are likely to be MAPs. Further research is needed to show whether AIR9-like proteins from trypanosomatid parasites, Trichomonas and other protozoans have microtubule binding sites.
Acknowledgements
This work was supported by a BBSRC Grant to Clive W. Lloyd. Miguel A. Andrade- Navarro is a Canada Research Chair in Bioinformatics.
Abbreviations
- AIR9
Auxin-Induced in Root Cultures 9
- IgG
g-immunoglobulin domain
- A9
domain IgG domain found in AIR9-like proteins
- GFP
green fluorescent protein
- LRR
leucine-rich repeat
- MAP
microtubule associated protein
- PPB
preprophase band
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4041
References
- 1.Buschmann H, Chan J, Sanchez-Pulido L, Andrade-Navarro MA, Doonan JH, Lloyd CW. Microtubule-associated AIR9 recognizes the cortical division site at preprophase and cell-plate insertion. Curr Biol. 2006;16:1938–1943. doi: 10.1016/j.cub.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 2.El-Sayed NM, Myler PJ, Blandin G, Berriman M, Crabtree J, Aggarwal G, Caler E, Renauld H, Worthey EA, Hertz-Fowler C, et al. Comparative genomics of Trypanosomatid parasitic protozoa. Science. 2005;309:404–409. doi: 10.1126/science.1112181. [DOI] [PubMed] [Google Scholar]
- 3.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucl Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlton JM, Hirt RP, Silva JC, Delcher AL, Schatz M, Zhao Q, Wortman JR, Bidwell SL, Alsmark UC, Besteiro S, et al. Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science. 2007;315:207–212. doi: 10.1126/science.1132894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simpson AG, Inagaki Y, Roger AJ. Comprehensive multigene phylogenies of excavate protists reveal the evolutionary positions of “primitive” eukaryotes. Mol Biol Evol. 2006;23:615–625. doi: 10.1093/molbev/msj068. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Ezpeleta N, Brinkmann H, Burey SC, Roure B, Burger G, Loffelhardt W, Bohnert HJ, Philippe H, Lang BF. Monophyly of primary photosynthetic eukaryotes: Green plants, red algae, and glaucophytes. Curr Biol. 2005;15:1325–1330. doi: 10.1016/j.cub.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 7.Embley TM, Martin W. Eukaryotic evolution, changes and challenges. Nature. 2006;440:623–630. doi: 10.1038/nature04546. [DOI] [PubMed] [Google Scholar]
- 8.Keeling PJ. Diversity and evolutionary history of plastids and their hosts. Am J Bot. 2004;91:1481–1493. doi: 10.3732/ajb.91.10.1481. [DOI] [PubMed] [Google Scholar]
- 9.Simpson AG, Roger AJ. The real ‘kingdoms’ of eukaryotes. Curr Biol. 2004;14:R693–R696. doi: 10.1016/j.cub.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 10.Leander BS. Did trypanosomatid parasites have photosynthetic ancestors? Trends Microbiol. 2004;12:251–258. doi: 10.1016/j.tim.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Waller RF, McConville MJ, McFadden GI. More plastids in human parasites? Trends Parasitol. 2004;20:54–57. doi: 10.1016/j.pt.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Hannaert V, Saavedra E, Duffieux F, Szikora JP, Rigden DJ, Michels PA, Opperdoes FR. Plant-like traits associated with metabolism of Trypanosoma parasites. Proc Natl Acad Sci USA. 2003;100:1067–1071. doi: 10.1073/pnas.0335769100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krepinsky K, Plaumann M, Martin W, Schnarrenberger C. Purification and cloning of chloroplast 6-phosphogluconate dehydrogenase from spinach: Cyanobacterial genes for chloroplast and cytosolic isoenzymes encoded in eukaryotic chromosomes. Eur J Biochem. 2001;268:2678–2686. doi: 10.1046/j.1432-1327.2001.02154.x. [DOI] [PubMed] [Google Scholar]
- 14.Martin W, Borst P. Secondary loss of chloroplasts in trypanosomes. Proc Natl Acad Sci USA. 2003;100:765–767. doi: 10.1073/pnas.0437776100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broadhead R, Dawe HR, Farr H, Griffiths S, Hart SR, Portman N, Shaw MK, Ginger ML, Gaskell SJ, McKean PG, Gull K. Flagellar motility is required for the viability of the bloodstream trypanosome. Nature. 2006;440:224–227. doi: 10.1038/nature04541. [DOI] [PubMed] [Google Scholar]
- 16.Richards TA, Cavalier-Smith T. Myosin domain evolution and the primary divergence of eukaryotes. Nature. 2005;436:1113–1118. doi: 10.1038/nature03949. [DOI] [PubMed] [Google Scholar]
- 17.Burki F, Pawlowski J. Monophyly of Rhizaria and multigene phylogeny of unicellular bikonts. Mol Biol Evol. 2006;23:1922–1930. doi: 10.1093/molbev/msl055. [DOI] [PubMed] [Google Scholar]