Abstract
Auxin plays a wide range of regulatory roles in diverse aspects of plant growth and developmental processes through a complex network of signaling interactions. In the May issue of Journal of Biological Chemistry, we have demonstrated that auxin homeostasis directly links growth regulation with stress adaptation responses through interactions with salicylic acid (SA) and abscisic acid (ABA) signals. In this signaling network, the endogenous auxin content is coordinately regulated through negative feedback by a group of auxin-inducible GH3 genes that encode auxin-conjugating enzymes. The Arabidopsis mutant wes1-D overexpressing a GH3 gene WES1 exhibits typical auxin-deficient traits, such as reduced growth and leaf curling, but is resistant to both biotic and abiotic stresses. In addition, various stress-regulated genes, including pathogenesis- related protein genes (PRs) and C-repeat/dehydration responsive element binding factor genes (CBFs), are up-regulated in the mutant. Consistent with these observations, WES1 is activated by pathogenic infections and abiotic stresses as well as by exogenous SA and ABA. We therefore propose that the WES1-mediated growth suppression would underlie the commonly observed symptoms of infected or stressed plants and provide a mechanism for auxin action in the fitness costs of induced resistance in plants.
Key Words: Arabidopsis, abscisic acid, auxin homeostasis, GH3, light, salicylic acid, stress adaptation
Numerous genes and signaling pathways have been characterized and the roles of several growth hormones, including SA, ABA, and jasmonic acid (JA), have been extensively studied in stress responses in plants. However, the underlying molecular mechanisms are largely unknown, primarily because of the complex interactions between multiple signaling pathways.1–4
Notably, it has recently been reported that auxin, which is widely recognized as a growth regulator in plants, also participates in stress responses.5–7 Frequently observed symptoms in infected or stressed plants are growth reduction and altered metabolism. In addition, auxin-regulated genes are affected in plants infected with pathogens.5,6 These observations suggest that auxin is involved in adaptive responses to biotic and abiotic stresses.
Auxin function is manifested, at least in part, at the transcriptional level by regulating a group of primary responsive genes, including Aux/IAAs, GH3s, and small auxin-up RNAs (SAURs).8 Particularly, all GH3-overexpressing mutants characterized so far, such as dfl1-D,9 dfl2-D,10 ydk1-D11 and wes1-D,12 exhibit severely reduced growth. This phenotype is well consistent with the biochemical activities of the GH3 proteins. The GH3 enzymes conjugate indole-3-acetic acid (IAA) to amino acids.13,14 We have recently demonstrated that the GH3-mediated growth regulation is closely linked to stress adaptation responses.12 The wes1-D mutant exhibits enhanced resistance to both biotic and abiotic stresses, and the PR and CBF genes are up-regulated in the mutant, strongly supporting that the WES1-mediated growth reduction is intimately associated with adaptive responses to environmental stresses.
Another feature of the WES1 gene is its light responsiveness. WES1 is induced by end-of-day far-red (EOD-FR) light, suggesting that WES1 may be regulated by the light-stable phytochrome B.15 The wes1-D mutant is phenotypically similar to axr2-1, an auxin-resistant mutant with a mutation in domain II of IAA7. However, whereas axr2-1 exhibits short hypocotyls in both light and darkness, the dwarfed phenotype of wes1-D occurs only in the light (Park et al., in preparation). When grown in darkness or under far-red light, the wes1-D hypocotyls were comparable to control hypocotyls. However, they were significantly shorter under red or blue light, suggesting a photomorphogenic role for WES1. In addition, all Arabidopsis GH3 genes characterized so far are responsive to light of particular wavelengths,9–11 suggesting that they are also involved in plant photomorphogenesis.
It is well known that phytochrome-mediated light signals are essential for PR induction and hypersensitive reaction (HR).16–18 These observations indicate that light also plays a critical role in SA-mediated disease resistance. This may be the reason why pathogen infection experiments are routinely carried out in darkness or in dim light. The WES1-mediated light signal is likely to be incorporated into the SA and ABA signaling pathways (Fig. 1). This idea is further supported by the repression of PRs and CBFs by auxin and the level of free SA in wes1-D.12 Further work will be required to determine unequivocally the physiological importance of light-SA interactions in the WES1-mediated signaling network. It would be an adaptive strategy that is activated preferentially in the light. Alternatively, it may be related with the rapid acclimation that is essential for plant fitness to natural environment.19
Figure 1.
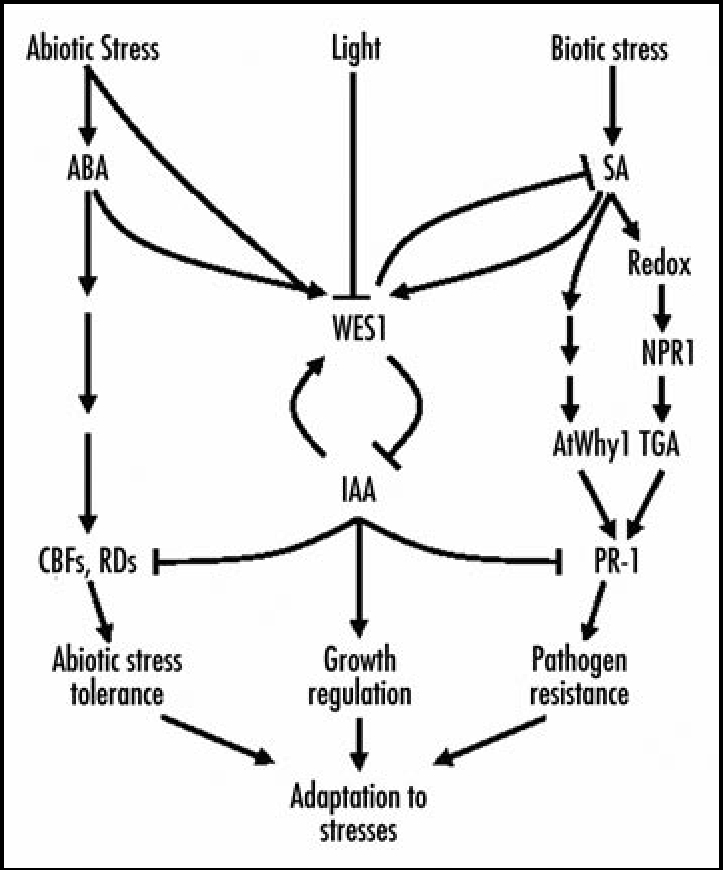
A proposed working model for WES1 function in stress adaptation responses. The endogenous auxin content is regulated by the WES1 enzyme through feedback regulation. WES1 is upregulated by environmental stresses as well as by SA and ABA, causing reduction of endogenous IAA and resultant growth retardation under stress conditions. It seems that light signals are also incorporated into the WES1-mediated signaling pathways, which may provide an adaptive advantage on stressed plants.
Acknowledgements
This work was supported by the Biogreen 21 (20050301034456) and National Research Laboratory (R0A-2005-000-10002-0) Programs and a grant from the Plant Signaling Network Research Center.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4069
References
- 1.Bohnert HJ, Nelson DE, Jensen RG. Adaptations to environmental stresses. Plant Cell. 1995;7:1099–1111. doi: 10.1105/tpc.7.7.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kunkel BN, Brooks DM. Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol. 2002;5:325–331. doi: 10.1016/s1369-5266(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 3.Gazzarrini S, McCourt P. Cross-talk in plant hormone signalling: What Arabidopsis mutants are telling us. Ann Bot. 2003;91:605–612. doi: 10.1093/aob/mcg064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katagiri F. A global view of defense gene expression regulation - A highly interconnected signaling network. Curr Opin Plant Biol. 2004;7:506–511. doi: 10.1016/j.pbi.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Tiryaki I, Staswick PE. An Arabidopsis mutant defective in jasmonate response is allelic to the auxin-signaling mutant axr1. Plant Physiol. 2002;130:887–894. doi: 10.1104/pp.005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowd C, Wilson IW, McFadden H. Gene expression profile changes in cotton root and hypocotyl tissues in response to infection with Fusarium oxysporum f. sp. vasinfectum. Mol Plant Microbe Interact. 2004;17:654–667. doi: 10.1094/MPMI.2004.17.6.654. [DOI] [PubMed] [Google Scholar]
- 7.Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, Voinnet O, Jones JDG. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science. 2006;312:436–439. doi: 10.1126/science.1126088. [DOI] [PubMed] [Google Scholar]
- 8.Hagen G, Guilfoyle T. Auxin-responsive gene expression: Genes, promoters, and regulatory factors. Plant Mol Biol. 2002;49:373–385. [PubMed] [Google Scholar]
- 9.Nakazawa M, Yabe N, Ichikawa T, Yamamoto YY, Yoshizumi T, Hasunuma K, Matsui M. DFL1, an auxin-responsive GH3 gene homologue, negatively regulates shoot cell elongation and lateral root formation, and positively regulates the light response of hypocotyl length. Plant J. 2001;25:213–221. doi: 10.1046/j.1365-313x.2001.00957.x. [DOI] [PubMed] [Google Scholar]
- 10.Takase T, Nakazawa M, Ishikawa A, Manabe K, Matsui M. DFL2, a new member of the Arabidopsis GH3 gene family, is involved in red light-specific hypocotyl elongation. Plant Cell Physiol. 2003;44:1071–1080. doi: 10.1093/pcp/pcg130. [DOI] [PubMed] [Google Scholar]
- 11.Takase T, Nakazawa M, Ishikawa A, Kawashima M, Ichikawa T, Takahashi N, Shimada H, Manabe K, Matsui M. ydk1-D, an auxin-responsive GH3 mutant that is involved in hypocotyl and root elongation. Plant J. 2004;37:471–483. doi: 10.1046/j.1365-313x.2003.01973.x. [DOI] [PubMed] [Google Scholar]
- 12.Park JE, Park JU, Kim YS, Staswick PE, Jeon J, Yun J, Kim SY, Kim J, Lee YH, Park CM. GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis. J Biol Chem. 2007 doi: 10.1074/jbc.M610524200. [DOI] [PubMed] [Google Scholar]
- 13.Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, Suza W. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic Acid. Plant Cell. 2005;17:616–627. doi: 10.1105/tpc.104.026690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staswick PE, Tiryaki I, Rowe ML. Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell. 2002;14:1405–1415. doi: 10.1105/tpc.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka S, Mochizuki N, Nagatani A. Expression of the AtGH3a gene, an Arabidopsis homologue of the soybean GH3 gene, is regulated by phytochrome B. Plant Cell Physiol. 2002;43:281–289. doi: 10.1093/pcp/pcf033. [DOI] [PubMed] [Google Scholar]
- 16.Genoud T, Millar AJ, Nishizawa N, Kay SA, Schäfer E, Nagatani A, Chua NH. An Arabidopsis mutant hypersensitive to red and far-red light signals. Plant Cell. 1998;10:889–904. doi: 10.1105/tpc.10.6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genoud T, Buchala AJ, Chua NH, Métraux JP. Phytochrome signalling modulates the SA-perceptive pathway in Arabidopsis. Plant J. 2002;31:87–95. doi: 10.1046/j.1365-313x.2002.01338.x. [DOI] [PubMed] [Google Scholar]
- 18.Zeier J, Pink B, Mueller MJ, Berger S. Light conditions influence specific defense responses in incompatible plant-pathogen interactions: Uncoupling systemic resistance from salicylic acid and PR-1 accumulation. Planta. 2004;219:673–683. doi: 10.1007/s00425-004-1272-z. [DOI] [PubMed] [Google Scholar]
- 19.Külheim C, Ågren J, Jansson S. Rapid regulation of light harvesting and plant fitness in the field. Science. 2003;297:91–93. doi: 10.1126/science.1072359. [DOI] [PubMed] [Google Scholar]


