Abstract
Stomata are microscopic pores on the plant epidermis that act as a major passage for the gas and water vapor exchange between a plant and the atmosphere. A pair of specialized guard cells work in concert to adjust pore size to maintain gas exchange while minimizing the water loss. The formation of stomata requires a series of cell-fate transitions from an initial meristemoid mother cell (MMC), to a stem-cell-like precursor meristemoid, to a guard mother cell (GMC), and finally to terminally-differentiated guard cells. Three closely-related Arabidopsis basic helix-loop-helix (bHLH) genes SPEECHLESS (SPCH), MUTE, and FAMA act sequentially at each key step to direct cell-fate transitions during stomatal development. In this addendum, we propose that a three-step relay of the three bHLHs establishes the molecular framework for stomatal differentiation. Specific expression patterns as well as protein domain structure and dimerization partners of each stomatal bHLH protein may determine the specific function as a key switch in each regulatory node.
Key Words: stomata, cell-type differentiation, asymmetric cell division, meristemoids, basic helix-loophelix (bHLH) protein, transcription factor
Introduction
Similar to many dicot plants, Arabidopsis guard cells differentiate via a series of stereotypical cell divisions, starting from an asymmetric entry division of an MMC that creates a meristemoid. The meristemoid possesses a transient stem-cell-like property and undergoes several rounds of asymmetric division that amplifies the number of surrounding cells. The meristemoid then differentiates into a round GMC, which divides symmetrically once to give rise to a pair of guard cells (Fig. 1A).1 While studies have revealed the presence of cell-cell signals required for proper orientation and density of stomata,2–5 genes that direct stomatal cell-type differentiation remained elusive.
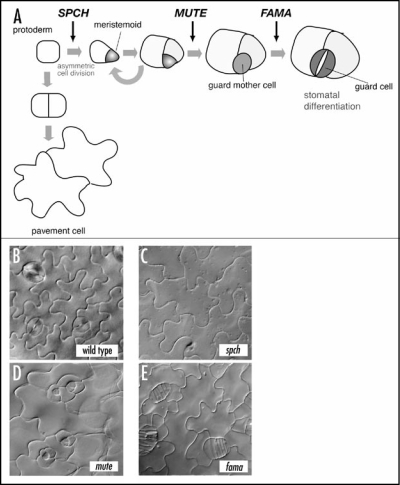
Figure 1.
(A) Schematic diagram of stomatal development and the site of each stomatal bHLH gene action (arrows). Modified from Pillitteri et al.6 (B–E). The leaf epidermal phenotype of wild type (B), spch (C), mute (D), and fama (E). All three mutants lack normal stomata seen in the wild type. spch produces epidermis solely made with pavement cells (C), mute produces rosette-like pattern with an arrested meristemoid at the center (D), and fama produces a stack of symmetrically divided GMC-like cells (E).
Three Stomatal BHLH Genes
Loss-of-function mutants of SPCH or MUTE give rise to aerial organs with no stomata (no “mouth” as implied by their names). However, their phenotypes are distinct. Unlike wild type (Fig. 1B), the epidermis of spch is solely made of jig-saw-puzzle-shaped pavement cells and lacks any stomatal cell lineage (Fig. 1C).6,7 In contrast, the initial asymmetric division and meristemoid formation occur normally in mute plants. However, mute meristemoids undergo excessive rounds of asymmetric divisions and subsequently abort instead of differentiating into GMCs. The mute epidermis is characterized by “islands” of asymmetric divisions that resemble an inward-spiral rosette pattern with an aborted, triangular meristemoid at the center (Fig. 1D). The third gene, FAMA (named after the “Goddess of rumor”), was previously identified.2 The fama mutation confers reiterative symmetric divisions of GMCs, forming abnormal rows of cells (as if they are “fake mouths”) but no mature guard cells (Fig. 1E). SPCH, MUTE, and FAMA direct three sequential steps of cell-fate transition during stomatal development: MMC to meristemoid by SPCH, meristemoid to GMC by MUTE, and GMC to guard cells by FAMA (Fig. 1A).
SPCH, MUTE, and FAMA encode three most closely-related basic-helix-loop-helix (bHLH) proteins among over 140 bHLH family members in Arabidopsis.6–9 This finding highlights a striking parallel between development of stomata and that of specialized cell types in animals, such as muscles and neurons. For example, during skeletal myogenesis, sequential activation of four closely-related myogenic bHLHs are required for skeletal muscle differentiation.10,11
Three-Step Relay
We propose a model by which SPCH, MUTE, and FAMA act sequentially at the key node of transcriptional cascades and together form a ‘three-step relay’. They do so by binding to regulatory elements of their target genes. In addition to their predicted molecular identities as DNA-binding transcription factors, two lines of evidence support this hypothesis. First, their spaciotemporal expression patterns correlate with each node: SPCH in protodermal cells, MUTE in a subset of meristemoids and early GMCs, and FAMA in GMCs and immature guard cells. The narrow windows of their promoter activities and the fact that no MUTE or FAMA expression was detected in spch mutant plants are highly indicative of the relay.6,7
Second, ectopic overexpression of the bHLHs conferred phenotypes specific to their respective regulatory nodes: SPCH overexpression creating a highly-divided epidermis with increased MMCs, MUTE overexpression conferring an epidermis solely composed of stomata, and FAMA overexpression generating massive clusters of single, unpaired guard cells. While MUTE is required for the transition of a meristemoid to GMC, ectopic MUTE overexpression is sufficient for stomatal differentiation. Therefore, once expressed, MUTE is capable of initiating downstream transcriptional cascades leading to terminal differentiation of stomata, perhaps by passing the ‘baton’ to FAMA. Consistently, ectopic overexpression of MUTE cannot override the absence of FAMA and results in a monstrous epidermis covered with massive clusters of GMC-like cells in the fama mutant background (our unpublished observation). The final outcome of the relay depends on the strength of key switch gene action. In the weak MUTE overexpressors, protodermal cells simultaneously execute both pavement- and guard cell gene expression programs. This leads to a formation of hybrid pavement/guard cells, in which a symmetric division plane accompanies a ‘faux’ pore.6 The relay model also explains the single, unpaired guard cells formed by the forced expression of FAMA.12 In this case, the initial cells skip the first two nodes of the relay and begin at the third node, maturation of guard cells. Further experiments will clarify whether the relay is connected directly (whether each bHLH directly upregulates the next player) or indirectly.
Similar Yet Unique
The distinct overexpression phenotypes by SPCH, MUTE and FAMA indicate that it is not simply their spaciotemporal expression patterns that specify their biological functions. Consistently, neither MUTE nor FAMA driven by the SPCH promoter rescued the spch mutant phenotype.7 The uniqueness of their functionality may lie in their amino-acid sequence and structural features. The bHLH domain mediates DNA binding through the basic stretch and homo-and heterodimerization through the HLH domain.13 The C-terminal region of SPCH, MUTE, and FAMA shows weak similarity to the ACT domain, a module known for the allosteric regulation of bacterial amino-acid biosynthetic enzymes.14 Recently, the C-terminal ACT-like domain of the Maize R bHLH protein was shown to mediate dimerization.15 Formation of homo/heterodimers with specific partners via HLH and ACT-like domains may provide specific functions to these three stomatal bHLH proteins.
Interestingly, MUTE lacks the N-terminal region juxtaposed to the bHLH domain. The truncation of this domain may be important for the functionality of MUTE as a key switch for meristemoid differentiation. This hypothesis is supported by the finding by Ohashi-Ito and Bergmann,12 who reported that ectopic overexpression of a truncated FAMA lacking the N-terminal stretch led to formation of massive stomatal clusters instead of single, unpaired guard cells.12 The neomorphic effect may be due to the truncated FAMA mimicking MUTE function. The results also suggest that the N-terminal region may be important for specific functions for FAMA and perhaps SPCH.
Future Perspectives
Stomatal development offers an excellent system to study cell division, cell-fate specification and cell-type differentiation, all of which lie at the heart of developmental biology. The discovery of SPCH, MUTE, and FAMA as closely-related bHLH proteins provides testable hypotheses to address their function as a molecular switch that drives cell state changes during stomatal development. Understanding specific domain functions of the stomatal bHLHs and identifying their downstream target genes will establish the molecular basis of the ‘three-step relay’. From such studies, one may be able to elucidate the molecular logic of complex cellular behavior supporting physiology and survival of the land plants.
Acknowledgements
The work in our laboratory has been supported by the grants from the US Department of Energy (DE-FG02-03ER15448), Japan Science & Technology Agency (CREST award), and by the US-National Science Foundation (IOB-0520548). K.U.T. is an affiliate faculty at the University of Washington, Institute of Stem Cell and Regenerative Medicine.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4072
References
- 1.Nadeau JA, Sack FD. Stomatal development in Arabidopsis. In: Somervile CR, Meyerowitz EM, editors. Arabidopsis Book. Rockville, MD: ASPB; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergmann DC, Lukowitz W, Somerville CR. Stomatal development and pattern controlled by a MAPKK kinase. Science. 2004;304:1494–1497. doi: 10.1126/science.1096014. [DOI] [PubMed] [Google Scholar]
- 3.Berger D, Altmann T. A subtilisin-like serine protease involved in the regulation of stomatal density and distribution in Arabidopsis thaliana. Genes Dev. 2000;14:1119–1131. [PMC free article] [PubMed] [Google Scholar]
- 4.Shpak ED, McAbee JM, Pillitteri LJ, Torii KU. Stomatal patterning and differentiation by synergistic interactions of receptor kinases. Science. 2005;309:290–293. doi: 10.1126/science.1109710. [DOI] [PubMed] [Google Scholar]
- 5.Nadeau JA, Sack FD. Control of stomatal distribution on the Arabidopsis leaf surface. Science. 2002;296:1697–1700. doi: 10.1126/science.1069596. [DOI] [PubMed] [Google Scholar]
- 6.Pillitteri LJ, Sloan DB, Bogenschutz NL, Torii KU. Termination of asymmetric cell division and differentiation of stomata. Nature. 2007;445:501–505. doi: 10.1038/nature05467. [DOI] [PubMed] [Google Scholar]
- 7.MacAlister CA, Ohashi-Ito K, Bergmann DC. Transcription factor control of asymmetric cell divisions that establish the stomatal lineage. Nature. 2007;445:537–540. doi: 10.1038/nature05491. [DOI] [PubMed] [Google Scholar]
- 8.Bailey PC, Martin C, Toledo-Ortiz G, Quail PH, Huq E, Heim MA, Jakoby M, Werber M, Weisshaar B. Update on the basic helix-loop-helix transcription factor gene family in Arabidopsis thaliana. Plant Cell. 2003;15:2497–2502. doi: 10.1105/tpc.151140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toledo-Ortiz G, Huq E, Quail PH. The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell. 2003;15:1749–1770. doi: 10.1105/tpc.013839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weintraub H. The MyoD family and myogenesis: Redundancy, networks, and thresholds. Cell. 1993;75:1241–1244. doi: 10.1016/0092-8674(93)90610-3. [DOI] [PubMed] [Google Scholar]
- 11.Olson EN. MyoD family: A paradigm for development? Genes Dev. 1990;4:1454–1461. doi: 10.1101/gad.4.9.1454. [DOI] [PubMed] [Google Scholar]
- 12.Ohashi-Ito K, Bergmann DC. Arabidopsis FAMA controls the final proliferation/differentiation switch during stomatal development. Plant Cell. 2006;18:2493–2505. doi: 10.1105/tpc.106.046136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murre C, McCaw PS, Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989;56:777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- 14.Chipman DM, Shaanan B. The ACT domain family. Curr Opin Struct Biol. 2001;11:694–700. doi: 10.1016/s0959-440x(01)00272-x. [DOI] [PubMed] [Google Scholar]
- 15.Feller A, Hernandez JM, Grotewold E. An ACT-like domain participates in the dimerization of several plant basic-helix-loop-helix transcription factors. J Biol Chem. 2006;281:28964–28974. doi: 10.1074/jbc.M603262200. [DOI] [PubMed] [Google Scholar]