Abstract
Allelopathy refers to plant-plant interference mediated mostly by plant released products of secondary metabolism. It was recently suggested that allelochamicals may influence growth of neighboring plants by induction of oxidative stress. We have focused on the role of reactive oxygen species (ROS) and phytohormons (ABA and ethylene) in the biochemical and molecular regulation of plant response to sunflower phytotoxins.
Key Words: ABA, allelopathy, ethylene, H2O2, reactive oxygen species, seed germination
Allelopathy Interaction
Allelopathy phenomenon was defined by Hans Molisch at the beginning of the XX century as the influence of one plant on another through releasing of chemicals into the environment.1 Allelochemicals in majority are secondary metabolites, released into the environment as exudates, volatiles and/or residues of plant tissue decomposition.2 The action of allelochemicals in target plant is diverse and affects a large number of biochemical reactions resulting in modifications of variety processes. The effects of allelochemicals action are detected at molecular, structural, biochemical, physiological and ecological levels of plant organization.3 Allelopathic compounds may induce a secondary oxidative stress manifested as enlarged production of reactive oxygen species (ROS).4 ROS are known to act as signaling molecules, regulating plant response to biotic (pathogen attack) and abiotic (drought, salinity, or heavy metals) stresses.5 In addition to, ROS are emerging as important regulators of plant development.6 Plant growth and development as well as plant response to stresses is controlled also by phytohormones. Hormonal signaling transduction depends on ROS production since; ROS have been implicated as second messengers in plant hormone responses.7 Ethylene and abscisic acid (ABA) are both regarded as typical stress hormones; they are also involved in regulation of seed dormancy and germination.8 Engagement of ROS into regulation of dormancy release and germination was reviewed by Bailly Ref. 9.
In last few years numerous articles relating to allelopathy interactions were published, although data on mechanism of action of identified allelochemicals are still exceptional.4,10 Here we summarize the hypothetical model of sunflower phytotoxins mode of action in germinating mustard (Sinapis alba) seeds.
Allelochemicals Induce Oxidative Stress and Hormonal Imbalance in Target Plants
It was suggested that, one of the effect of allelochemicals on target plant may be uncontrolled production and accumulation of ROS, accompanied by activation of cellular antioxidant system.4 In our previous reports we described the mode of action of sunflower foliar extract during mustard seed germination.11,12 We demonstrated that sunflower phytotoxins inhibited mustard seed germination13 not only by induction of oxidative stress,12 but also by restriction in reserve mobilization.11 Sunflower extract induced an oxidative burst expressed as an increased H2O2 concentration in germinating mustard seeds.12 It correlated well with enhanced MDA level12 and electrolyte leakage.13 Therefore it may be suggested that loss of seed viability in reaction to sunflower allelochemicals is associated with increased lipid peroxidation. Similar response was observed by Bais et al.10 demonstrating that (-)-catechin elicits a burst of ROS in Arabidopsis thaliana roots. Extract of leaves and stems of beauty-berry (Callicarpa accuminata) increased free radicals level, catalase (CAT) activity and membrane damage in tomato roots.14 The exposure of cucumber (Cucumis sativus) roots to ferulic and p-coumaric acids increased both H2O2 level and peroxidase (POD) activity.15 Cucumber root extracts increased CAT, superoxide dismutase (SOD) and POD activity in cucumber roots.16 In our experiment foliar sunflower extract stimulated both SOD and CAT activity. Enhancement of CAT activity was associated with synthesis of CAT 5 isoform, identified by western blot analysis. We concluded that although the cellular antioxidant system is activated rapidly after treatment with sunflower extract it does not function sufficiently to avoid some cellular damage.12 Bais et al10 demonstrated that (-)-catechin lead to cell death of target plant roots via ROS by ROS-triggered Ca2+-signalling cascade causing cellular pH decrease and allelochemical-induced genome-wide changes ingene expression pattern.17 Therefore we suggest that in allelopathy stress induced by sunflower phytotoxins, ROS (H2O2) may also act as signaling molecules leading to changes in hormonal balance during germination of mustard seeds. To verify this suggestion, we measured synthesis of two stress hormones (ABA and ethylene) in mustard seeds imbibed in sunflower phytotoxins. ABA concentration in treated seeds was doubled comparing to the seeds imbibed in water.18 The high level of ABA is typical for dormant seeds, therefore, we may suspect that sunflower phytotoxins blocks metabolic activity of the embryo and seeds became artificially dormant (still viable but unable to germinate). In the contrast, ethylene emission by mustard seeds was strongly repressed by sunflower extract.18 Ethylene biosynthesis is controlled by two key enzymes ACC synthase (ACS) and ACC oxidase (ACO).19 ACO activity decreased as exposition to sunflower allelochemicals extended. The addition of exogenous ACC (ACO substrate) does not enhanced ethylene biosynthesis.18 It may indicate that the limitation in its evolution was the consequence of ACO deactivation associated with enhanced membrane deterioration, resulting probably from increased lipid peroxidation by ROS. ACS activity increased transiently after short period of seed imbibition in the presence of sunflower allelochemicals. However, the prolonged treatment by sunflower phytotoxins resulted in decrease of ACS activity.18 Ethylene transduction cascade involves MAPKs.20 It is suggested that ROS (mainly H2O2) act upstream of MAPK pathways.21 Thus modifications of MAPKs activity can lead to alteration in ethylene response.
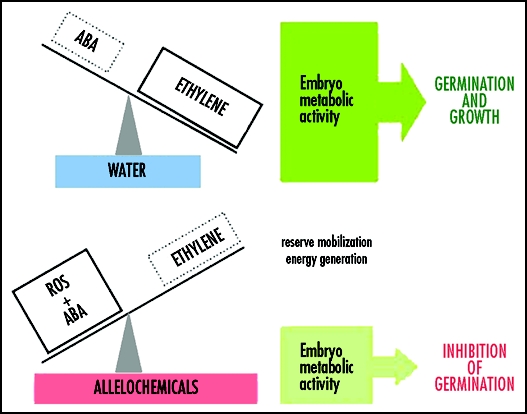
In conclusion, sunflower allelochemicals induce disturbances in hormonal balance between ABA and ethylene in germinating mustard seeds. Oxidative burst leading to accumulation of ROS results in enhanced membrane peroxidation. Low ethylene concentration, resulting from inhibition of ACS and ACO activity may enhance seed sensitivity to ABA, which concentration increased in seeds treated by sunflower extract. The disturbances in phytohormone levels leads to decreasing metabolic activity of the embryo and blocking its germination and growth (Fig. 1).
Figure 1.
Schematic of signaling pathways including ROS, ABA and ethylene regulation of germination of mustard seeds imbibed in water or sunflower foliar extract. Sunflower allelochemicals enhanced ROS production, leading to enlargement in membrane deterioration.12 Declined ACS and ACO activity resulted in decreased ethylene synthesis.18 Perturbation in hormonal balance: high concentration of ABA and low ethylene evolution made seeds “artificially” dormant. Low energy supply due to suppression in reserve (lipids and proteins) mobilization inhibition ICL - isocitrate lyase and endopeptidase activity) slowed down energy (ATP) generation11 in the catabolic phase of germination and finally blocked radicle protrusion.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4116
References
- 1.Molish H. Der Einflus einer Pflanze auf die andere, Allelopathie. Jena, Germany: Verlag von Gustav Fisher; 1937. (Ger). English translation. In: La Fleur LJ, Mallik MAB, eds. Influence of One Plant on Another. Jodhpur: Scientific Publishers, 2001. [Google Scholar]
- 2.Weston LA, Duke SO. Weed and crop allelopathy. Critical Rev Plant Sci. 2003;22:367–389. [Google Scholar]
- 3.Gniazdowska A, Bogatek R. Allelopathic interaction between plants: Multi site action of allelochemicals. Acta Physiol Plant. 2005;27:395–408. [Google Scholar]
- 4.Weir T, Park SW, Vivianco JM. Biochemical and physiological mechanisms mediated by allelochemicals. Curr Opin Plant Biol. 2004;7:472–479. doi: 10.1016/j.pbi.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17:1866–1875. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gapper C, Dolan L. Control of plant development by reactive oxygen species. Plant Physiol. 2006;141:341–345. doi: 10.1104/pp.106.079079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwak JM, Nguyen V, Schroeder JI. The role of reactive oxygen species in hormonal responses. Plant Physiol. 2006;141:323–329. doi: 10.1104/pp.106.079004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kucera B, Cohn MA, Leubner-Metzger G. Plant hormone interactions during seed dormancy release and germination. Seed Sci Res. 2005;15:281–307. [Google Scholar]
- 9.Bailly C. Active oxygen species and antioxidants in seed biology. Seed Sci Res. 2004;14:93–107. [Google Scholar]
- 10.Bais HP, Vepechedu R, Gilroy S, Callaway RM, Vivanco JM. Allelopathy and exotic plant invasion: From molecules and genes to species interactions. Science. 2003;301:1377–1380. doi: 10.1126/science.1083245. [DOI] [PubMed] [Google Scholar]
- 11.Kupidłowska E, Gniazdowska A, Stepien J, Corbineau F, Vinel D, Skoczowski A, Janeczko A, Bogatek R. Impact of sunflower (Helianthus annuus L.) extracts upon reserve mobilization and energy metabolism in germinating mustard (Sinapis alba L.) seeds. J Chem Ecol. 2006;32:2569–2583. doi: 10.1007/s10886-006-9183-z. [DOI] [PubMed] [Google Scholar]
- 12.Oracz K, Bailly C, Gniazdowska A, Come D, Corbineau F, Bogatek R. Induction of oxidative stress by sunflower phytotoxins in germinating mustard seeds. J Chem Ecol. 2007;33:251–264. doi: 10.1007/s10886-006-9222-9. [DOI] [PubMed] [Google Scholar]
- 13.Bogatek R, Gniazdowska A, Zakrzewska W, Oracz K, Gawronski SW. Allelopathic effects of sunflower extracts on mustard seed germination and seedling growth. Biol Plant. 2006;50:156–158. [Google Scholar]
- 14.Cruz-Ortega R, Ayala-Cordero G, Anaya AL. Allelochemical stress produced by aqueous leachate of Callicarpa acuminata: Effects on roots of bean, maize, and tomato. Physiol Plant. 2002;116:20–27. doi: 10.1034/j.1399-3054.2002.1160103.x. [DOI] [PubMed] [Google Scholar]
- 15.Politycka B, Kozłowska M, Mielcarz B. Cell wall peroxidases in cucumber roots induced by phenolic allelochemicals. Allelopathy J. 2004;13:29–36. [Google Scholar]
- 16.Yu JQ, Ye SF, Zhang MF, Hu WH. Effects of root exudates and aqueous root extracts of cucumber (Cucumis sativus) and allelochemicals, on photosynthesis and antioxidant enzymes in cucumber. Biochem System Eol. 2003;31:129–139. [Google Scholar]
- 17.Callaway RM, Vivianco JM. Invasion of plants into native communities using the underground superhighway. In: Harper JDI, An M, Wu H, Kent JH, editors. Proceedings of the 4 th World Congress on Allelopathy, Wagga Wagga, NSW, Australia, Charles Stuart University, International Allelopathy Society. 2005. pp. 50–56. [Google Scholar]
- 18.Gniazdowska A, Oracz K, Bogatek R. Phytotoxic effect of sunflower (Helianthus annuus L.) to hormonal balance (ABA: Ethylene) in germinating mustard (Sinapis alba L.) seeds. Allelopathy J. 2007;19:215–226. [Google Scholar]
- 19.Petruzzelli L, Corragio I, Leubner-Metzger G. Ethylene promotes ethylene biosynthesis during pea seed germination by positive feedback regulation of 1-aminocyclo-propane-1-carboxylic acid oxidase. Planta. 2000;211:144–149. doi: 10.1007/s004250000274. [DOI] [PubMed] [Google Scholar]
- 20.Chang C. Ethylene signaling: The MAPK module has been finally landed. Trends Plant Sci. 2003;8:365–368. doi: 10.1016/S1360-1385(03)00156-0. [DOI] [PubMed] [Google Scholar]
- 21.Pitzschke A, Hirt H. Mitogen-activated protein kinases and reactive oxygen species signaling in plants. Plant Physiol. 2006;141:351–356. doi: 10.1104/pp.106.079160. [DOI] [PMC free article] [PubMed] [Google Scholar]