Abstract
The use of electric fields for measuring cell and tissue properties has a long history. However, the exploration of the use of electric fields in tissue engineering is only very recent. A review is given of the various methods by which electric fields may be used in tissue engineering, concentrating on the assembly of artificial tissues from its component cells using electrokinetics. A comparison is made of electrokinetic techniques with other physical cell manipulation techniques which can be used in the construction of artificial tissues.
Key words: tissue engineering, electric field, microenvironment, electrokinetics, dielectrophoresis, polarity
Introduction
The investigation of the electrical properties of biological materials and their applications has a long history. Tissue engineering, in contrast, is a relatively more recent field of investigation, and the exploration of the use of electric fields for characterizing or actively making artificial tissues has only just begun. However, as research in this highly competitive field is expanding rapidly, a review of the use of electric fields in tissue engineering appears very timely.
The main applications of electric fields in tissue engineering are in the characterisation of artificial tissues and its component cells, and the formation of artificial tissue-like materials, either by assisting in the formation of the artificial extracellular matrix (e.g., the formation of scaffolds by electrospinning), or the micromanipulation of the cells themselves using electric fields. Of further potential interest in tissue engineering are also the biological effects of the electric fields. In this review we will briefly discuss all these topics, concentrating on the manipulation of cells using electrokinetic techniques.
Characterization of Cells and Tissues Using Electrical Techniques
The electrical properties of tissues are mainly determined by their capacitance and conductance. Both are frequency-dependent, and the frequency range over which the electrical properties of tissues can be measured ranges from subHz to the microwave range (Giga-TeraHz). A large variety of techniques have been developed for the measurement of the electrical properties of tissues, contact as well as non-contact methods. Reviews of the electrical properties of natural tissues have been given by various authors.1–3 Measurements on natural tissues can give information on for example the orientation of the cells in the tissue, tissue fat content, moisture content, tissue damage through thermal or other effects, tissue freshness—or time since death.4–6 Also, pronounced differences exist between the electrical properties of some cancerous and non-cancerous tissues.7
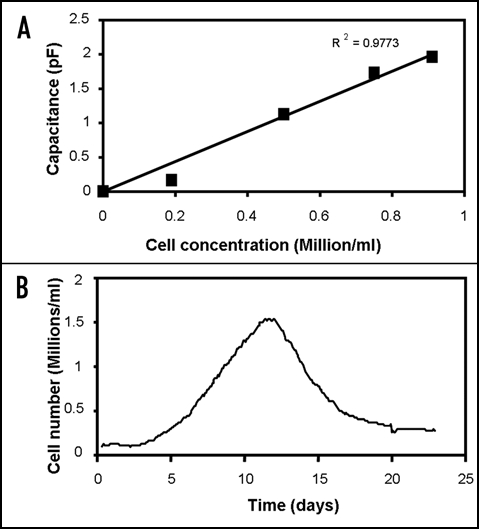
An important potential application of dielectric measurements on engineered tissues is the on-line and continuous measurement of the cell concentration and its distribution within a tissue construct.8,9 An example of the on-line measurement of cell concentration in a tissue construct is shown in Figure 1.
Figure 1.
Capacitance measurement of cell concentrations in tissue constructs. (A) Capacitance at 0.4 MHz of fibroblasts cells at different concentrations in a fibrin gel, showing a linear relation between capacitance and cell concentration. (B) Constantly monitoring the capacitance at 0.4 MHz allows the cell concentration in the artificial tissue to be followed in time. The gel was inoculated with 100.000 human fibroblast cells/ml in DMEM growth medium with 10% fetal calf serum. Capacitance measurements were performed with an Aber Instruments model 220 Biomass Monitor (Aber Instruments, Aberystwyth, UK).
Of interest in tissue engineering are also impedance measurements on adherent cells growing as confluent cell layers over an electrode surface.10 Because of the non-conductive nature of the cell membrane the impedance across a confluent cell layer can be very high, and small changes in cell properties can be picked up.
Electrospinning
Electrospinning typically involves the application of a high voltage (usually several kV) to a polymer solution or polymer melt between a conductive die (usually a capillary) and a counterelectrode. The high electrical field generated between the capillary and the counterelectrode causes the formation of a fine fluid jet, from which nanofibers are formed which can be used as scaffolds.11
Cell Manipulation Using Electric Fields, and its Use in the Construction of Artificial Cell Assemblies
DC electric fields.
Under most circumstances cells have a net negative charge, and when a DC electric field is applied to a cell suspension the cells are readily moved by electrophoresis. By generating positive voltages at micropatterned electrodes electrophoresis can be used to attract and pattern cells.12–13 The electric field that is needed to electrophorese cells is quite large. This large external DC electric field elicits very high electric fields across the cell membrane, which can adversely affect cell viability. The other disadvantage of the use of DC electric fields for patterning cells is that other effects such as electro-osmotic flow of the medium and heating effects give rise to flows near the electrodes. This makes it difficult to control cell movement, whilst excessive heating can also affect cell viability. Reducing the medium conductivity by replacing (conductive) salts by (nonconductive) sugars can alleviate these problems to some extent.
AC electric fields.
Although electro-osmotic and heating effects also occur in AC electric fields, these effects significantly reduce with increasing frequency, and at frequencies over 1 MHz they can often be neglected. AC electric fields across the membrane also decrease with increasing frequency, and thus AC electric fields are preferable for cell manipulation over DC fields. Unlike DC electric fields, particle movement in (radio frequency) AC electric fields is dominated by dipole effects rather than the net surface charge. Different polarization effects dominate at different frequencies. In the MHz range cell dipoles are dominantly formed by interfacial polarization effects at the cell membrane. The cell membrane has a much lower dielectric constant and is much less conductive than the suspending medium and the cytoplasm, causing charge accumulation at the membrane and the formation of a large dipole moment in an externally applied electric field in this frequency range. The interaction between the dipole induced by the electric field and the electric field itself can lead to a variety of effects. These effects include electro-orientation, electro-rotation, dielectrophoresis and electrostretching.14–16
Electro-orientation.
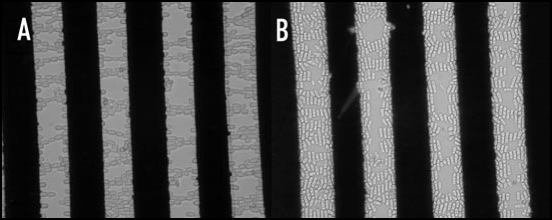
Electro-orientation of a particle in an electric field occurs if the induced dipole moment along one axis of the particle is stronger than that along the other axes. The dipole moment of many particles, including many cells, has a different frequency-dependency along different axes. This can cause cells to change their orientation if the frequency is changed.17–19 An example is shown in Figure 2, in which (fission) yeast cells are shown to change their orientation from along the electric field lines at 1 MHz to perpendicular to the electric field lines at around 50 MHz. Electro-orientation of animal cells is feasible; this clearly has potential applications in tissue engineering as many tissues contain oriented cells.19
Figure 2.
Electro-orientation of (fission) yeast cells. The electrodes used had a spacing and width of 40 µm; the applied voltage was 10 V peak-to-peak. (A) Orientation along electric field lines at 1 MHz; (B) Orientation perpendicular to electric field at 50 MHz.
Electro-rotation.
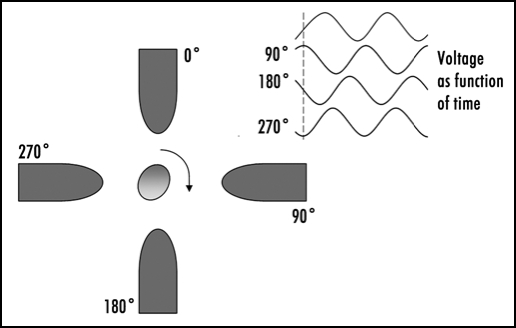
Electro-rotation refers to the rotation of particles in electric fields. Although rotation of cells can be observed in static electric fields, more reproducible electrorotation can be obtained if the cell is subjected to a rotating electric field. Such rotating electric fields can easily be generated by applying four signals that are 90 degree phase-shifted relative to each other to four electrodes surrounding a cell20(see also Fig. 3). The rate and direction in which a cell rotates is frequency-dependent; this can be used to obtain information about the properties of a cell's substructures such as the permeability or wrinkliness of the cell membrane, or the conductivity of the cytoplasm.21 The rate at which a particle rotates at a given frequency is easily controlled by changing the voltage. Controlling the orientation of cells by electrorotation could be useful in tissue engineering for orienting polarized cells in specific directions.
Figure 3.
Outline of the principle of an electro-rotation setup, with the phases of the electric field signal applied to each electrode. The electro-rotation method can be used for orienting polarized cells in any desired direction.
Dielectrophoresis.
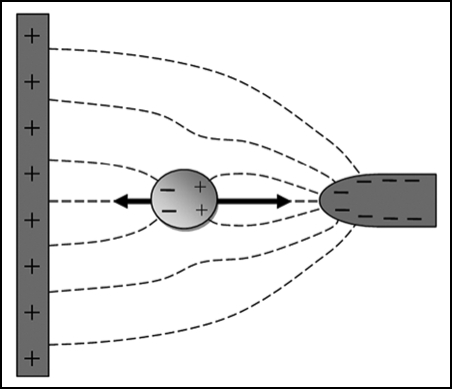
The term “dielectrophoresis” refers to the induced lateral movement of particles in non-uniform electric fields. The principle of dielectrophoresis is shown in Figure 4. Dielectrophoresis is readily observed when cells are suspended in low conductivity buffers, and electric fields are applied to the cells using microelectrodes of a size in the range of 20–200 µm, and AC signals with a frequency in the range 10 kHz–100 MHz and voltages of 2–20 V peak-to-peak. The magnitude and direction of the dielectrophoretic force is dependent on the frequency of the electric field. For example, in low conductivity iso-osmotic buffers mammalian cells show negative dielectrophoresis (away from high field regions) at low frequencies around 10 kHz; positive dielectrophoresis (towards high field regions) is shown at frequencies around 1 MHz.
Figure 4.
Principle of dielectrophoresis. The dashed lines represent electric field lines. A dipole is induced by the electric field in the particle, and a force is exerted on the induced charges on both sides of the particle. Because the electric field strength is stronger near the smaller electrode on the right than near the larger electrode on the left the particle will experience a net force, pulling the particle towards the smaller electrode. If the charges on the electrodes are reversed the distribution of charges in the dipole will also reverse; dielectrophoresis will therefore occur in both AC and DC electric fields. If the surrounding medium is more polarisable than the particle the net force will be in the opposite direction (negative dielectrophoresis rather than the positive dielectrophoresis depicted in Figure 4).
The construction of tissue-like materials using dielectrophoresis was first investigated in 1972 by Pohl,22,23 who showed that rod-shaped bacterial cells could be attracted to high field regions around a cylindrical electrode by dielectrophoresis, forming a tissue-like material. The orientation within the tissue could be controlled by changing the frequency.23 Although it took a long time before this work was taken up again, in recent years research in this area has gained momentum, with work being done on the construction of tissue-like materials from a variety of cell types.25–29 Dielectrophoretic spectra of very different cell types are very similar,30 and the method can be employed with essentially any cell type—microbial, animal or plant—or in fact non-cellular material.31–33 Both negative and positive dielectrophoresis can be used,32,33 single cell manipulation is possible,34–38 and aggregate sizes can range from single cells to hundreds of microns.28,39–43 Because microelectrode arrays can be made in which different electrodes are addressed at different times using signals of different voltages and frequencies, and different cell types can be introduced at different times, complex two- and three-dimensional patterns of different cell types can be created using dielectrophoresis. This makes it a useful method for the creation of defined cell assemblies in which the same or different cell types interact.44 Examples of such assemblies to date have included artificial neural networks,45–48 artificial liver constructs,49,50 and artificial embryonic and haematopoietic stem cells microenvironments. 39,42 An example of the latter is shown in Figure 5, in which a multicellular three-dimensional aggregate is shown consisting of three different cell types, which together form an artificial microniche for a haematopoietic stem cell.
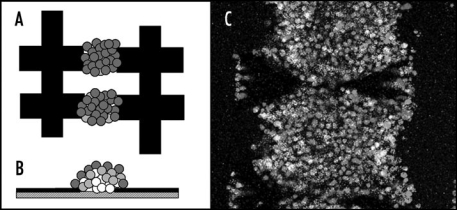
Figure 5.
Artificial haematopoietic stem cell microniches created using dielectrophoresis. (A) Top view; (B) side view; (C) actual aggregate. The aggregate shown is made from successive layers of osteoblast (bottom), stromal (middle) and Jurkat (top) cells, attracted between interdigitated oppositely castellated electrodes. The aggregates, which are approximately 500 µm across, mimic the osteoblast haematopoietic stem cell niche in bone marrow. Vascular haematopoietic stem cell niches, which include endothelial cells, can be created using a similar approach.51
Although the research in this area is increasingly concentrating on mammalian tissues, work on the creation of artificial cell microenvironments using dielectrophoresis has also included microbial systems. This has included studies of quorum sensing (community effects) and metabolite exchange in bacterial aggregates.52–54
If positive DEP is used to attract cells to high field regions between the electrodes, then—even though the attractive electric forces decline as a function of height—the dielectrophoretic forces are strong enough to put down several layers of cells, at heights over 150 µm.39–42 At the high cell concentrations used in a typical experiment with dielectrophoresis the interaction between the dipole formed by the individual cells is strong. This often result in the attraction of the dipoles (cells) to each other in the electric field, which then form what are called “pearlchains.” Aggregates of cells at the high field regions (in the case of positive DEP) are often dominated by pearlchain formation rather than the movement of individual cells by DEP.40 When the cells are assembled together by the positive DEP force the cells are in direct contact with each other; this will enable direct cell-cell communication to occur within the assembled aggregates. Also, when assembled the cells have a tendency to adhere to each other. If the cells have been forced into direct contact with each other for some time the adherent forces between the cells are often strong enough for the electric field to be removed without the aggregate immediately falling apart into its component cells. This effect is only temporary, but significantly simplifies any subsequent steps such as the introduction of a gel for further cell immobilisation.42
Once the cells have been guided to their final position with dielectrophoresis a further immobilization step is needed to keep the cells in place. For two-dimensional aggregates, in which the cells are in direct contact with the (electrode) surface, surface modification with agents such as fibronectin is often used to promote surface attachment. A variety of gels have also been used, both for 2- and 3-D aggregates; these have included photopolymerisible gels,26,43,44 agarose44 and fibrin gels. Other approaches include the use of crosslinking agents.29,53,54
Stronger forces can be exerted on the cells with positive dielectrophoresis than with negative dielectrophoresis. However, manipulation of cells with positive DEP necessarily involves the use of low-conductivity iso-osmotic buffers which may negatively influence cell viability. Higher conductivities can be used when employing negative dielectrophoresis, but the force is much smaller. An interesting approach to overcome this problem is the use of beads of conductive hydrogels containing cells;50 the gels allow the conductivity of the medium to be lowered to levels where positive dielectrophoresis occurs, whilst the cells are still in a highly conductive environment formed by the gel.
The microelectrode arrays used for the AC electrokinetic construction of tissue-like materials are often made using photolithography. Typically, these arrays have covered an area of a few square cm. However, a method developed by Abidin et al.,55 shown in Figure 6, in which textiles containing parallel metal wires are used to attract cells by dielectrophoresis, could make it possible to create tissue-like materials over large surface areas or volumes.
Figure 6.
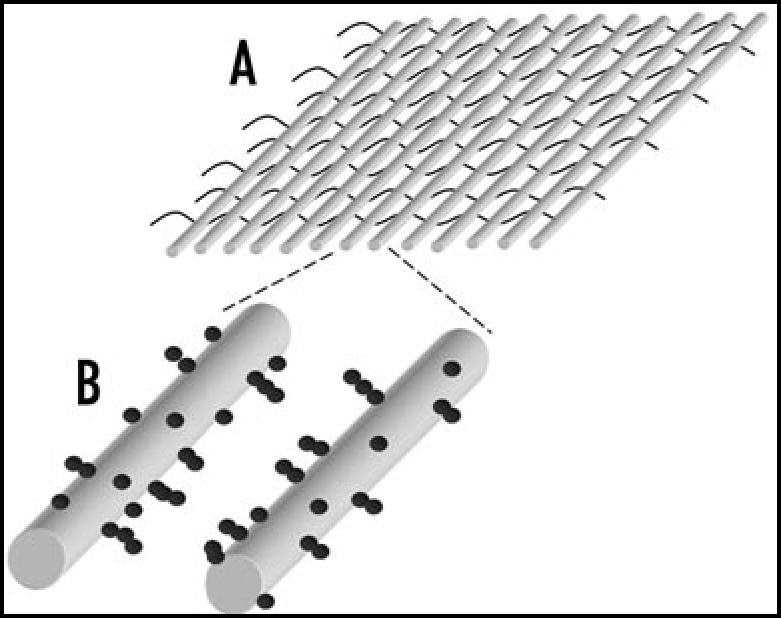
Textiles can be created which can be used for constructing cellular arrays using dielectrophoresis on large scale.55
Electrostretching of cells.
When a cell forms a dipole the charge accumulation occurs at opposite ends of the cell (see also Fig. 4). The electrical forces will therefore be strongest where these charges have accumulated, and at very high electric field strength this can lead to stretching of the cells.56 Mechanical forces are well-known to affect cell behavior in tissues, and electrostretching may be a method by which mechanical forces are indirectly exerted on cells. However, before electrostretching could be an effective method for exerting mechanical forces on cells in tissues many technical problems would still have to be overcome. In particular, it is difficult to generate sufficiently high electric field strength across tissues, especially at high medium conductivities, whilst maintaining cell viability.
Biological Response of Cells to Electric Fields
In the previous section the response of the cells to the electric fields was essentially passive; whether a cell moved or not was determined by the physical properties of the cell such as its surface charge or the presence of an insulating membrane surrounding a highly conducting cytoplasm. However, cells can also actively respond to electric fields. We will explore some examples of active responses by cells to electric field exposure, and how this may be used in tissue engineering.
DC electric fields.
An excellent review of DC electric fields and their role in biological systems has been given by McCaig et al.57 DC electric fields are well known very important in biology; for example in membrane potentials, ion flow through protein channels, action potentials in the nervous system. However, lesser known is that steady, long-lasting DC currents that occur on a multicellular scale are also of importance. Such steady, long-lasting DC currents have been shown to be generated by a large variety of biological systems, and are of importance during, amongst others, embryo formation and wound healing. It is therefore no surprise that externally applied DC currents have been found to have many biological effects, and that many cells respond to steady DC electric fields.57,58 Such responses include galvanotaxis,57,59 which involves cells actively moving to either the cathode or the anode, and electrotropism,57,60 which involves the orientation of cells in electric fields.
Galvanotaxis and electrotropism could be used in tissue engineering, to guide cells to predefined positions, or orient cells in particular directions (e.g., neurons). Steady DC electric fields are also involved in the maintenance of cell polarity in natural tissues, in particular epithelial cells. There clearly is an opportunity to control cell polarity in artificial tissues using externally applied DC electric fields.
AC electric fields.
Investigation of the response of cells to AC electromagnetic fields has concentrated on effects related to exposure to low-frequency (e.g., power lines) or high-frequency electromagnetic fields (e.g., microwave cookers, mobile phones). At the low powers involved it is often very difficult to ascertain whether the exposure to electromagnetic fields has an effect or not, and the very limited effects observed to date have certainly not been sufficient to seek a role for low power/low strength AC electric fields in tissue engineering.
High strength AC electric fields, in contrast, are well-known to have various effects on cells. These effects range from heating to membrane disruption and death. Exposure of cells to high strength AC electric fields is in fact an effective method for the sterilization of materials.61 Exposure of cells to electric field pulses of low duration can temporarily induce small holes in the membrane, which close rapidly without affecting cell viability to a large extend; this effect has been used in electroporation and electrofusion.62,63 During tissue assembly with dielectrophoresis or related AC electrokinetic techniques the cellular membranes are often exposed to electric fields close to a value of 30–40 kV/m. This can kill the cells.42,64 However, investigations of the long-term exposure of cells to sublethal electric fields of below this magnitude in growth medium have found no or very minor electric field effects;65,66 when cells are exposed to sublethal electric fields in low conductivity iso-osmotic buffers it is difficult to separate the effects of the buffer from the effects of the electric field; again, any effects by the electric fields itself are likely to be minor.
Comparison of the Use of Electrical Fields in Tissue Engineering with Other Physical Force Field-Based Techniques
No review of the use of electric field in tissue engineering would be complete if no reference was made to other physical cell micromanipulation techniques. In recent years a whole raft of techniques have been developed which can be used to micro-engineer artificial tissues from its components.67,68 Although techniques such as inkjet technology and related free-flow techniques, microfluidic approaches and surface patterning techniques (topological or chemical) are also of interest, to keep the review short a comparison will be made of cell manipulation techniques based on physical force fields only.
Apart from the electrical techniques,24,69 optical,70–73 ultrasound74,75 and magnetic techniques76,77 are currently also under development for their use in assembling artificial tissues. A comparison of the techniques is given in Table 1. Electric-field based techniques often need to be employed in low conductivity buffers to be fully effective; this is not the case with the other techniques, which can work directly in growth medium. The procedures used for assembling tissues with electric fields are also often harsher compared with the other techniques, although in practice to date we have fortunately always found that conditions can be found which are acceptable for the cells. Electric-field based approaches do not have the spatial resolution of laser tweezers-based approaches, but the number of cells that can be manipulated at the same time using electric fields is only limited by the size of the electrode array, whilst with laser tweezer approaches the number of cells that can be manipulated is very limited. Ultrasound based approaches do not have good spatial resolution. A strong disadvantage of magnetic approaches is that cells are not normally magnetisable, and that to be able to manipulate cells using magnetic fields they have to be made magnetisable by forcing the cells to take up para-magnetic materials. Such an alteration of the cells is not needed by the other methods.
Table 1.
Comparison of different physical force field-based techniques for cell manipulation
| Physical force field | Typical equipment needed | Spatial resolution achievable | Effect of field on cell viability | Manipulation directly in growth medium? | Number of cells that can be manipulated simultaneously | Typical volume handled |
| Optical | Microscope with NIR laser and motorized stage | Very high, nanometers | Some heating | Yes | Small (typically single cells; 100's is possible) | pL |
| Electrical | Microelectrodes; frequency generator | Good; typically microns | Heating; breakdown of membrane integrity at high fields | Difficult | Single cells as well as large numbers (millions) | µL-mL |
| Magnetic | Magnet | Similar to electrical techniques | Cells have to be made magnetisable; otherwise negligible | Yes | Single cells as well as large numbers (millions) | µL-mL |
| Ultrasound | Resonance chamber with piezo-electric transducer with resonance frequency in range 1 MHz–10 MHz | Typically low, tens of microns | Small | Yes | Typically large numbers (millions) | mL |
Conclusions
Electrical fields are and will be increasingly used in tissue engineering. This is due not only to the fact that electrical fields are useful in the characterization and constructing of artificial tissue, both also because electricity plays important roles in living systems. One area in which electrical fields may increasingly find a role is in the construction of artificial tissues from their component cells. Electrical cell manipulation techniques, however, will have to compete with other physical cell manipulation techniques. Each physical cell manipulation method has its advantages and disadvantages. Electrical techniques are, however, particularly useful. With electrical techniques it is possible to do experiments with relatively simple equipment. In addition, control is straightforward, good spatial resolution can be achieved, and many cells can be handled simultaneously without their prior modification.
Acknowledgements
I would like to thank staff at Intercytex for useful discussions and the permission to publish work on capacitance measurements in artificial tissues, and the Royal Academy of Engineering for providing funds for an Industrial Secondment at Intercytex. I would also like to thank Dr. A.M. Buckle for many useful discussions and the use of her facilities for some of the experimental work, and Dr. Hatfield and McGowan for the use of the microelectrode manufacturing facilities. Special thanks go to A. McGilchrist and B. Alp, and A. Sebastian, for the use of their pictures on electro-orientation and haematopoietic stem cell microniches, respectively. This work has received funding from the BBSRC (grant BB/D002850/1: the Hematon project) and the Wellcome Trust.
Abbreviations
- AC
alternating current
- DC
direct current
Footnotes
Previously published online as an Organogenesis E-publication: http://www.landesbioscience.com/journals/organogenesis/article/5799
References
- 1.Pethig R, Kell DB. The passive electrical properties of biological systems: their significance in physiology, biophysics and biotechnology. Phys Med Biol. 1987;32:933–970. doi: 10.1088/0031-9155/32/8/001. [DOI] [PubMed] [Google Scholar]
- 2.Gabriel S, Lau RW, Gabriel C. The dielectric properties of biological tissues, 2. Measurements in the frequency range 10 Hz to 20 GHz. Phys Med Biol. 1996;41:2251–2269. doi: 10.1088/0031-9155/41/11/002. [DOI] [PubMed] [Google Scholar]
- 3.Foster KR, Schwan HP. Dielectric properties of tissues and biological materials: a critical review. CRC Crit Rev Biomed Eng. 1989;17:25–104. [PubMed] [Google Scholar]
- 4.Alanen E, Lahtinen T, Nuutinen J. Measurement of dielectric properties of subcutaneous fat with open-ended coaxial sensors. Phys Med Biol. 1998;43:475–485. doi: 10.1088/0031-9155/43/3/001. [DOI] [PubMed] [Google Scholar]
- 5.Raicu V, Saibara T, Irimajiri A. Multifrequency method for dielectric monitoring of cold-preserved organs. Phys Med Biol. 2000;45:1397–1407. doi: 10.1088/0031-9155/45/5/321. [DOI] [PubMed] [Google Scholar]
- 6.Querido D. Temperature-correction of abdominal impedance: improved relationship between impedance and postmortem interval. Forensic Science International. 2000;109:39–50. doi: 10.1016/s0379-0738(99)00217-0. [DOI] [PubMed] [Google Scholar]
- 7.Joines WT, Zhang Y, Li CX, Jirtle RL. The measured electrical properties of normal and malignant human tissues from 50 to 900 MHz. Medical Physics. 1994;21:547–550. doi: 10.1118/1.597312. [DOI] [PubMed] [Google Scholar]
- 8.Kyle AH, Chan CTO, Minchinton AI. Characterization of three-dimensional tissue cultures using electrical impedance spectroscopy. Biophys J. 1999;76:2640–2648. doi: 10.1016/S0006-3495(99)77416-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris CM, Todd RW, Bungard SJ, Lovitt RW, Morris JG, Kell DB. Dielectric permittivity of microbial suspensions at radio frequencies: a novel method for the real-time estimation of microbial biomass. Enzyme Microb Technol. 1987;9:181–186. [Google Scholar]
- 10.Boudriot U, Dersch R, Greiner A, Wendorff JH. Electrospinning approaches toward scaffold engineering—a brief overview. Artificial Organs. 2006;30:785–792. doi: 10.1111/j.1525-1594.2006.00301.x. [DOI] [PubMed] [Google Scholar]
- 11.Wegener J, Keese CR, Giaever I. Electric cell-substrate impedance sensing (ECIS) as a noninvasive means to monitor the kinetics of cell spreading to artificial surfaces. Exp Cell Res. 2000;259:158–166. doi: 10.1006/excr.2000.4919. [DOI] [PubMed] [Google Scholar]
- 12.Ozkan M, Pisanic T, Scheel J, Barlow C, Esener S, Bhatia SN. Electro-optical platform for the manipulation of life cells. Langmuir. 2003;19:1532–1538. [Google Scholar]
- 13.Ozkan M, Ozkan CS, Kibar O, Wang MM, Bhatia S, Esener SC. Heterogeneous integration through electrokinetic migration. IEEE Eng Med Biol. 2001;20:144–151. doi: 10.1109/51.982286. [DOI] [PubMed] [Google Scholar]
- 14.Hughes MP. Nanoelectromechanics in engineering and biology. Boca Raton: CRC Press; 2003. [Google Scholar]
- 15.Markx GH, Davey CL. The dielectric properties of biological cells at radio-frequencies: applications in biotechnology. Enzyme Microb Technol. 1999;25:161–171. [Google Scholar]
- 16.Jones TB. Electromechanics of particles. Cambridge: Cambridge University Press; 1995. Cambridge. [Google Scholar]
- 17.Miller RD, Jones TB. Electro-orientation of ellipsoidal erythrocytes. Biophys J. 1993;64:1588–1595. doi: 10.1016/S0006-3495(93)81529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markx GH, Alp B, McGilchrist A. Electro-orientation of Schizosaccharomyces pombe in high conductivity media. J Microb Methods. 2002;50:55–62. doi: 10.1016/s0167-7012(02)00012-x. [DOI] [PubMed] [Google Scholar]
- 19.Yang M, Zhang X. Electrical assisted patterning of cardiac myocytes with controlled macroscopic anisotropy using a microfluidic dielectrophoresis chip. Sensors Actuators A. 2007;35:73–79. [Google Scholar]
- 20.Arnold WM, Zimmermann U. Electro-rotation—development of a technique for dielectric measurements on individual cells and particles. J Electrostat. 1988;21:151–191. [Google Scholar]
- 21.Wang XB, Huang Y, Gascoyne PRC, Becker FF, Hoelzel R, Pethig R. Changes in Friend murine erythroleukaemia cell membranes during induced differentiation determined by electrorotation. Biochim Biophys Acta. 1994;1193:330–344. doi: 10.1016/0005-2736(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 22.Pohl HA. Dielectrophoresis. Cambridge: Cambridge University Press; 1978. [Google Scholar]
- 23.Pohl HA. Electrical forming of masses of living cells. J Colloid Interface Sci. 1972;39:437–438. [Google Scholar]
- 24.Markx GH, Buckle AM. Encyclopedia of Biomaterials and Biomedical Engineering. New York: Taylor & Francis; 2005. Tissue engineering: AC electrokinetics. [Google Scholar]
- 25.Matsue T, Matsumoto N, Uchida I. Rapid micropatterning of living cells by repulsive dielectrophoretic force. Electrochim Acta. 1997;42:3251–3256. [Google Scholar]
- 26.Alp B, Stephens GM, Markx GH. Formation of artificial, structured microbial consortia (ASMC) by dielectrophoresis. Enzyme Microb Technol. 2002;31:35–43. [Google Scholar]
- 27.Alp B, Andrews JS, Mason VP, Wolowacz R, Markx GH. Building structured biomaterials using AC electrokinetics. IEEE Eng Med Biol. 2003;22:91–97. doi: 10.1109/memb.2003.1266052. [DOI] [PubMed] [Google Scholar]
- 28.Albrecht DR, Sah RL, Bhatia SN. Geometric and material determinants of patterning efficiency by dielectrophoresis. Biophys J. 2004;87:2131–2147. doi: 10.1529/biophysj.104.039511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verduzco Luque CE, Alp B, Stephens GM, Markx GH. Construction of biofilms with defined internal architecture using dielectrophoresis and flocculation. Biotechnol Bioeng. 2003;83:39–44. doi: 10.1002/bit.10646. [DOI] [PubMed] [Google Scholar]
- 30.Pethig R, Markx GH. Applications of dielectrophoresis in biotechnology. Trends Biotechnol. 1997;15:426–432. doi: 10.1016/S0167-7799(97)01096-2. [DOI] [PubMed] [Google Scholar]
- 31.Velev OD, Bhatt KH. On-chip micromanipulation and assembly of colloidal particles by electric fields. Soft Matter. 2006;2:738–750. doi: 10.1039/b605052b. [DOI] [PubMed] [Google Scholar]
- 32.Pethig R, Huang Y, Wang XB, Burt JPH. Positive and negative dielectrophoretic collection of colloidal particles using interdigitated castellated electrodes. J Phys D: Appl Phys. 1992;25:881–888. [Google Scholar]
- 33.Wang XB, Huang Y, Burt JPH, Markx GH, Pethig R. Selective dielectrophoretic confinement of bioparticles in potential energy wells. J Phys D: Appl Phys. 1993;26:1278–1285. [Google Scholar]
- 34.Müller T, Pfennig A, Klein P, Gradl G, Jäger M, Schnelle T. The potential of dielectrophoresis for single-cell experiments. IEEE Eng Med Biol Mag. 2003:51–61. doi: 10.1109/memb.2003.1266047. [DOI] [PubMed] [Google Scholar]
- 35.Manaresi N, Romani A, Medoro G, Altomare L, Leonardi A, Tartagni M, Guerrieri R. A CMOS chip for individual cell manipulation and detection. IEEE Journal of Solid-State Circuits. 2003;38:2297–2305. [Google Scholar]
- 36.Gray DS, Tan JL, Voldman J, Chen CS. Dielectrophoretic registration of living cells to a microelectrode array. Biosens Bioelectron. 2004;19:1765–1774. doi: 10.1016/j.bios.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 37.Rosenthal A, Voldman J. Dielectrophoretic traps for single-particle patterning. Biophys J. 2005;88:2193–2205. doi: 10.1529/biophysj.104.049684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mittal N, Rosenthal A, Voldman J. NDEP microwells for single-cell patterning in physiological media. Lab Chip. 2007;7:1146–1153. doi: 10.1039/b706342c. [DOI] [PubMed] [Google Scholar]
- 39.Sebastian A, Buckle AM, Markx GH. Formation of multilayer aggregates of mammalian cells by dielectrophoresis. J Micromech Microeng. 2006;16:1769–1777. [Google Scholar]
- 40.Sebastian A, Venkatesh AG, Markx GH. Tissue engineering with electric fields: Investigation of the shape of mammalian cell aggregates formed at interdigitated oppositely castellated electrodes. Electrophoresis. 2007;28:3821–3828. doi: 10.1002/elps.200700019. [DOI] [PubMed] [Google Scholar]
- 41.Venkatesh AG, Markx GH. On the height of cell aggregates formed by positive dielectrophoresis. J Phys D: Appl Phys. 2007;40:106–113. [Google Scholar]
- 42.Sebastian A, Buckle AM, Markx GH. Tissue engineering with electric fields: immobilisation of mammalian cells in multilayer aggregates using dielectrophoresis. Biotechnol Bioeng. 2007;98:694–700. doi: 10.1002/bit.21416. [DOI] [PubMed] [Google Scholar]
- 43.Albrecht DR, Tsang VL, Sah RL, Bhatia SN. Photo- and electropatterning of hydrogel-encapsulated living cell arrays. Lab Chip. 2005;5:111–118. doi: 10.1039/b406953f. [DOI] [PubMed] [Google Scholar]
- 44.Albrecht DR, Underhill GH, Wassermann TB, Sah RL, Bhatia SN. Probing the role of multicellular organization in three-dimensional microenvironments. Nature Methods. 2006;3:369–375. doi: 10.1038/nmeth873. [DOI] [PubMed] [Google Scholar]
- 45.Heida T, Rutten WLC, Marani E. Dielectrophoretic trapping of dissociated fetal cortical rat neurons. IEEE Trans Biomed Eng. 2001;48:921–930. doi: 10.1109/10.936368. [DOI] [PubMed] [Google Scholar]
- 46.Heida T, Vulto P, Rutten WL, Marani E. Viability of dielectrophoretically trapped neural cortical cells in culture. J Neurosci Methods. 2001;110:37–44. doi: 10.1016/s0165-0270(01)00414-9. [DOI] [PubMed] [Google Scholar]
- 47.Prasad S, Yang M, Zhang X, Ozkan CS, Ozkan M. Electric field assisted patterning of neuronal networks for the study of brain functions. Biomed Microdevices. 2003;5:125–137. [Google Scholar]
- 48.Yu Z, Xiang G, Pan L, Huang L, Yu Z, Xing W, Cheng J. Negative dielectrophoretic force assisted construction of ordered neuronal networks on cell positioning bioelectronic chips. Biomed Microdev. 2004;6:311–324. doi: 10.1023/B:BMMD.0000048563.58129.76. [DOI] [PubMed] [Google Scholar]
- 49.Ho CT, Lin RZ, Chang WY, Chang HY, Liu CH. Rapid heterogeneous liver-cell on-chip patterning via the enhanced field-induced dielectrophoresis trap. Lab Chip. 2006;6:724–734. doi: 10.1039/b602036d. [DOI] [PubMed] [Google Scholar]
- 50.Albrecht DR, Underhill GH, Mendelson A, Bhatia SN. Multiphase electropatterning of cells and biomaterials. Lab Chip. 2007;7:702–709. doi: 10.1039/b701306j. [DOI] [PubMed] [Google Scholar]
- 51.Yin T, Li L. The stem cell niches in bone. The Journal of Clinical Investigation. 2006;116:1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Markx GH, Andrews JS, Mason VP. Towards microbial tissue engineering? Trends Biotechnol. 2004;22:417–422. doi: 10.1016/j.tibtech.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 53.Mason VP, Markx GH, Thompson IP, Andrews JS, Manefield M. Colonial architecture in mixed species assemblages affects AHL mediated gene expression. FEMS Microbiology Letters. 2005;244:121–127. doi: 10.1016/j.femsle.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 54.Andrews JS, Mason VP, Thompson IP, Stephens GM, Markx GH. Construction of artificially structured microbial consortia (ASMC) using dielectrophoresis: Examining bacterial interactions via metabolic intermediates within environmental biofilms. J Microb Methods. 2006;64:96–106. doi: 10.1016/j.mimet.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 55.Abidin ZZ, Downes L, Markx GH. Large scale dielectrophoretic construction of biofilms using textile technology. Biotechnol Bioeng. 2007;96:1222–1225. doi: 10.1002/bit.21228. [DOI] [PubMed] [Google Scholar]
- 56.Sukhorukov VL, Mussauer H, Zimmermann U. The effect of electrical deformation forces on the electropermeabilization of erythrocyte membranes in low- and high-conductivity media. J Membrane Biol. 1998;163:235–245. doi: 10.1007/s002329900387. [DOI] [PubMed] [Google Scholar]
- 57.McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling cell behavior electrically: current views and future potential. Physiol Rev. 2005;85:943–978. doi: 10.1152/physrev.00020.2004. [DOI] [PubMed] [Google Scholar]
- 58.Zhao M, Song B, Pu J, Wada T, Redi B, Tai GP, Wang F, Guo AH, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, Penninger JM. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–460. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
- 59.Sun S, Wise J, Cho M. Human fibroblast migration in three-dimensional collagen gel in response to noninvasive electrical stimulus—I. Characterization of induced three-dimensional cell movement. Tissue Engineering. 2004;10:1548–1557. doi: 10.1089/ten.2004.10.1548. [DOI] [PubMed] [Google Scholar]
- 60.Sun S, Titushkin I, Cho M. Regulation of mesemchymal stem cell adhesion and orientation in 3D collagen scaffold by electrical stimulus. Bioelectrochemistry. 2006;69:133–141. doi: 10.1016/j.bioelechem.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 61.Jeyamkondan S, Jayas DS, Holley RA. Pulsed electric field processing of foods: A review. J Food Protection. 1999;62:1088–1096. doi: 10.4315/0362-028x-62.9.1088. [DOI] [PubMed] [Google Scholar]
- 62.Neil GA, Zimmermann U. Electrofusion. Methods in Enzymology. 1993;221:171–196. doi: 10.1016/0076-6879(93)20082-e. [DOI] [PubMed] [Google Scholar]
- 63.Ho SY, Mittal GS. Electroporation of cell membranes: A review. Crit Rev Biotechnol. 1996;16:349–362. doi: 10.3109/07388559609147426. [DOI] [PubMed] [Google Scholar]
- 64.Menachery A, Pethig R. Controlling cell destruction using dielectrophoretic forces. IEE Proc Nanobiotechnol. 2005;152:145–149. doi: 10.1049/ip-nbt:20050010. [DOI] [PubMed] [Google Scholar]
- 65.Fuhr G, Glasser H, Müller T, Schnelle T. Cell manipulation and cultivation under ac electric field influence in highly conductive culture media. Biochim Biophys Acta. 1994;1201:353–360. doi: 10.1016/0304-4165(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 66.Archer S, Li TT, Evans AT, Britland ST, Morgan H. Cell reactions to dielectrophoretic manipulation. Biochem Biophys Res Comm. 1999;257:687–698. doi: 10.1006/bbrc.1999.0445. [DOI] [PubMed] [Google Scholar]
- 67.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci USA. 2006;103:2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park H, Cannizzaro C, Vunjak-Novakovic G, Langer R, Vacanti CA, Farokhzad OC. Nanofabrication and microfabrication of functional materials for tissue engineering. Tissue Engineering. 2007;13:1867–1877. doi: 10.1089/ten.2006.0198. [DOI] [PubMed] [Google Scholar]
- 69.Voldman J. Electrical forces for microscale cell manipulation. Ann Rev Biomed Eng. 2006;8:425–454. doi: 10.1146/annurev.bioeng.8.061505.095739. [DOI] [PubMed] [Google Scholar]
- 70.Grier DG. A revolution in optical manipulation. Nature. 2003;424:810–816. doi: 10.1038/nature01935. [DOI] [PubMed] [Google Scholar]
- 71.Birkbeck AL, Flynn RA, Ozkan M, Song DQ, Gross M, Esener SC. VCSEL Arrays as micromanipulators in chip-based biosystems. Biomedical Microdevices. 2003;5:47–54. [Google Scholar]
- 72.Odde DJ, Renn MJ. Laser-guided direct writing of living cells. Biotechnol Bioeng. 2000;67:312–318. doi: 10.1002/(sici)1097-0290(20000205)67:3<312::aid-bit7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 73.Odde DJ, Renn MJ. Laser-guided direct writing for applications in biotechnology. Trends Biotechnol. 1999;17:385–389. doi: 10.1016/s0167-7799(99)01355-4. [DOI] [PubMed] [Google Scholar]
- 74.Bazou D, Dowthwaite GP, Khan IM, Archer CW, Ralphs JR, Coakley WT. Gap junctional intercellular communication and cytoskeletal organization in chondrocytes in suspension in an ultrasound trap. Mol Membrane Biol. 2006;23:195–205. doi: 10.1080/09687860600555906. [DOI] [PubMed] [Google Scholar]
- 75.Bazou D, Kuznetsova LA, Coakley WT. Physical environment of 2-D animal cell aggregates formed in a short pathlength ultrasound standing wave trap. Ultrasound Med Biol. 2005;31:423–430. doi: 10.1016/j.ultrasmedbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 76.Ino K, Ito A, Honda H. Cell patterning using magnetite nanoparticles and magnetic force. Biotechnol Bioeng. 2007;97:1309–1317. doi: 10.1002/bit.21322. [DOI] [PubMed] [Google Scholar]
- 77.Tanase M, Felton EJ, Gray DS, Hultgren A, Chen CS, Reich DH. Assembly of multicellular constructs and microarrays of cells using magnetic nanowires. Lab Chip. 2005;5:598–605. doi: 10.1039/b500243e. [DOI] [PubMed] [Google Scholar]