Abstract
Action potentials in higher plants are believed to be the information carriers in intercellular and intracellular communication in the presence of an environmental stressor. Plant electrophysiologists have recorded long distance electrical signaling in higher plants during the last two hundred years. Reproducing the duration, speed of propagation, and the shape of the action potential is challenging. Early measurements revealed that the speed of action potential propagation in plants is extremely slow - from 0.1 mm/s to 20 cm/s, although many faster plant responses to stress have been recorded as well. We hypothesized that this discrepancy is most likely due to the artifacts of aliasing from slow registration systems. In this study, we employ real time measurements using modern data acquisition techniques to detect ultra fast action potentials in green plants induced by localized heat stress. Thermal shock or heat stress is the most common environmental stress. Based on more sophisticated measuring techniques, we show that plants transmit solitary waves and that the speed of action potential propagation in green plants is similar to the speed of action potentials in mammalians, varying from a few meters per second up to 105 m/s. Possible pathways for electrical signal propagation in vascular plants are discussed.
Key words: Action potential, plant electrophysiology, electrical signaling, localized heat stress, excitability
Introduction
Excitability is a specific property that allows cells, tissues and organs to alter their internal condition and external reactions under the impact of various environmental factors, referred to as irritants. According to Goldsworthy,1 electrochemical signals resembling nerve impulses exist in plants at all evolutionary levels. Action potentials (APs) in higher plants can be generated in response to mechanical, physical or chemical external irritants.2–9
The existence of electrical signaling in plants has been known for more than two centuries.10–14 As the field progressed, AP velocities were measured using a variety of methods. These first measurements revealed that the velocities of APs range from 0.1 mm/s to 20 cm/s.12,15–18 These slow AP velocities are similar to the speeds of diffusion of 0.6–4.0 mm/s as seen in the phloem19 and 25 cm/s in the xylem.20 The previous measurements are significantly slower than those recorded in animal neurons. The existence of carnivorous or “motorized” plants and their fast movements cast certain doubts on the accuracy of these measurements. Sachs21 discussed the paradox between slow speeds of AP propagation registered by Burdon-Sanderson12 in the Venus flytrap and ultra fast plant mechanical responses during the trap closing, because such slow action potentials12 can be a result of the Venus flytrap closure, but not its cause.
We hypothesized that the discrepancies that exist between the traditional AP velocities measured in plants and the more recent recorded velocities are due to aliasing. The Shannon sampling theorem, a fundamental rule of sampled data systems, states that the input signal must be sampled at a rate greater than twice the highest frequency component in the signal. The critical sampling rate is called the Nyquist rate. Mathematically, fs/2 > fmax, where fs is the sampling frequency and fmax is the maximum frequency of the signal being sampled. Violating the Shannon sampling theorem is considered undersampling and results in aliasing. Aliasing does not infer that the “Sampled Value” is erroneous, but rather it means that the inferred time dependence of a series of samples is distorted. Due to this limitation, all data presented in this paper were collected on high speed data acquisition systems.
The electrophysiological signaling in higher plants strongly depends on environmental conditions.22–24 Plants are exposed to a diverse array of continuously varying perturbations, including variations of temperature.25–26 Plants generate different types of extracellular electrical events in connection to environmental stress.27 Thermal shock is induced in plants, animals, insects, bacteria, and fungi by drastic changes in temperature. Thermal shock alters gene expression and leads to increased heat tolerance in a wide range of organisms. The response of many organisms to elevated temperature has been characterized and described as the heat shock response. In plants, heat stress stimulates the production of heat shock proteins (HSP).28 HSPs assist plants in the adaptation to and tolerance of extreme temperatures.29 None of the mechanisms by which higher plants perceive abiotic stresses has been elucidated. Progress in this crucial area will substantially advance our knowledge of stress initiated signal transduction events. The main goal of this study was to investigate in real time the electrical signaling in higher plants induced by a thermal wounding.
Results and Discussion
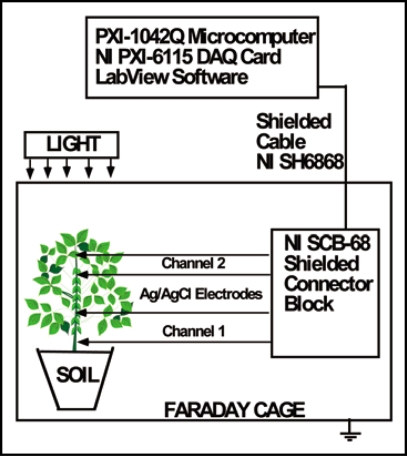
We employed a novel PXI system to measure electrical signaling in plants induced by localized thermal stress. This experimental approach is illustrated in Figure 1. We approximated the velocity of AP propagation as the distance between two channels (pairs of Ag/AgCl electrodes) divided by time between maximum values of AP registered by these channels.
Figure 1.
Experimental setup for measuring electrical signals in soybean.
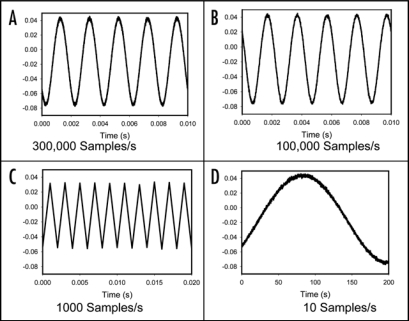
When electrochemical signals are measured, it is extremely important to take into consideration of the sampling rate, which determines how often the measurement device samples an incoming analog signal. According to the sampling theorem, the original analog signal must be adequately sampled in order to be properly represented by the digital signal, which is acquired. If the sampling rate is too slow, the rapid changes in the original signal in between any two consecutive samples cannot be accurately recorded. As a result, higher frequency components of the original signal will be misrepresented as lower frequencies (aliasing). In signal processing, this problem is known as aliasing. According to the Nyquist Criterion, the sampling frequency must be at least twice the bandwidth of the signal to avoid aliasing. As illustrated in Figure 2A and B, a sinusoidal signal with 500 Hz frequency can be uniquely reconstructed from the digitized signal when sampled at a rate of 300,000 samples/s or 100,000 samples/s, a sampling rate well above the Nyquist frequency limit of 1,000 samples/s. However, when the sampling frequency is at the Nyquist frequency limit, the distortion begins as shown in Figure 2D. According to Figure 2D, it is obviously impossible to reconstruct the original signal when it is under sampled at any frequency below the Nyquist Criterion limit, such as a sampling rate of 10 samples/s. During the last 125 years, plant electrophysiologists have measured action potential in plants for with extremely slow registration systems and without anti-aliasing low pass filters. Due to electronic effects of aliasing and different time constant τ = RC of analog voltmeters, different authors published different speeds of the action potential propagation (Table 1), different amplitudes and duration time of action potentials even for the same plants such as tomato plant. It is hypothesized that these discrepancies in the results among different groups of researchers were caused by aliasing or large time constants of high input impedance analog or digital voltmeters.
Figure 2.
Reconstructed 500 Hz sinusoidal signal from the digitized signal sampled at (a) 300,000 samples/second, (b) 100,000 samples/second, (c) the Nyquist rate of 1,000 samples/second; (d) aliased 500 Hz signal due to undersampling at 10 samples/second.
Table 1.
Electrical signals in higher plants induced by thermal stimuli
| Plant | Stimulus | Potential Amplitude, mV | Potential Duration, s | Potential Speed, cm/s | Potential Length, cm | Low - Pass Anti-Aliasing Filter | Physiological Effect | References | |
| 1 | Tomato (Lycopersicon esculentum Mill.) | Localized heat | AP: 49 | 60–300 | 2.5–4.0 | 150–1200 | No | Induction of pin gene expression | 18 |
| 2 | Tomato (Lycopersicon esculentum Mill.) | Localized heat | AP: 79 | 10 | 0.2 | 2 | No | Induction of pin gene expression | 30 |
| 3 | Tomato (Lycopersicon esculentum cv. Heinz 1350) | Localized heat | AP: No VP: 40 |
No ∼60 |
No 0.4 |
No ∼24 |
No | Induction of pin gene expression | 31 |
| 4 | Tomato (Lycopersicon esculentum cv. Heinz 1350) | Localized heat | AP: No VP: 74 |
No >1800 |
No ? |
No ? |
No | Induction of pin gene expression | 24 |
| 5 | Wheat (Triticum durum Desf., cv. Iva) | Localized heat | AP: No VP: ? |
No ? |
No ? |
No ? |
No | Induction of stomata activity | 16 |
| 6 | Tobacco (Nicotiana tabacum cv. Samsun) | Localized heat | EEP | >3600 | 1.5–2.0 | 5400–7200 | No | Stomata closure, reductions in the rate of transpiration and CO2 assimilation | 32 |
| 7 | Sunflower (Heliantis annuns) | Localized heat | AP: 18.5–24.4 | 14.7–18.4 | 1.3–2.7 | 24–39.7 | No | Action potential is the carrier of information | 33 |
| 8 | Bean (Phaseolus multiflorus Willd.) | Localized heat | AP: 22.3–27.0 | 16.3–26.1 | 0.5 | 8.2–13.05 | No | Action potential is the carrier of information | 33 |
| 9 | Buckwheat (Fagopyrum sagittaeum Gilib.) | Localized heat | AP: 17.1–19.5 | 16.9–24.6 | 0.9–1.2 | 22.1–20.3 | No | Action potential is the carrier of information | 33 |
| 10 | Pumpkin (Cucurbita pepo L.) | Localized heat | AP: 8.5–17.9 | 12.2–16.4 | 0.7–1.0 | 11.5–12.2 | No | Action potential is the carrier of information | 33 |
| 11 | Pumpkin (Cucurbita pepo L.) | Gradual cooling | AP: 18 (13°C) 117 (26°C) |
30–60 | ? | ? | No | Increase in cold resistance | 34 |
| Pumpkin (Cucurbita pepo L.) | Gradual cooling | ∼120 mV | ? | ? | ? | No | Increase in cold resistance | 35 | |
| 12 | Mimosa pudica | Cooling with ice water | AP: 150 | 8 | 2–3 | 16–24 | No | Leaf movements | 36 |
| 13 | Mimosa pudica | Localized heat | AP: 80–100 | 120–300 | 0.4–0.8 | 48–240 | No | Leaf movements | 37 |
| 14 | Hibiscus rosasinensis L. (Malvaceae) | Localized heat | AP: No VP: 100 |
>150 | 1.3 | >195 | No | Respiration change; reduced metabolite concentrations in the ovary | 38 |
| 15 | Hibiscus rosasinensis L. (Malvaceae) | Cooling with ice water | AP: 100 | 2–3 | 2 | 4–6 | No | Respiration change; reduced metabolite concentrations in the ovary | 38 |
| 16 | Aloe vera | Localized heat | AP: 40 | 0.01 | 6,700 | 67 | Yes | 39 | |
| 17 | Aloe vera | Ice | AP: 20–35 | 0.003 | 13,200 | 40 | Yes | 39 | |
| 18 | Soybean (Glycine max (L.) Merrill) | Localized heat | AP: 130 | 0.001 | 10,500 | 10.5 | Yes | Present work |
AP, action potential; VP, variation potential; EEP, extracellular electrical potential.
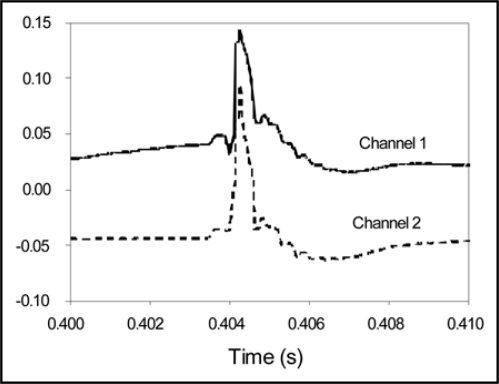
Localized heat wounding induces high speed electrical signals in vascular plants, specifically in soybean (Fig. 3). Our results show that a single application of heat induces fast action potentials in soybean plants (105.5 m/s, s.d. = 5.6 m/s, n = 9). These high speed APs are comparable to the recorded AP velocity in the neuron. Figure 3 shows that the duration of a solitary wave in soybean is about 1 ms. A localized thermal stress applied for 1 second generates only one action potential which is transmitted from the injured leaf through stem to roots.
Figure 3.
Action potentials induced in a soybean plant by thermal shock (flame). Sampling rate is 100,000 scans per second and distance between channels is 2 cm.
Plants possess many chemical aspects similar to the neuromotoric system in animals.40 For example, plants employ neurotransmitters such as acetylcholine, dopamine, noradrenalin, serotonin and histamine,40 cellular messengers like calmodulin,40 the cellular motors actin and myosin, and voltage-gated ion channels.9 Plants also utilize a variety of sensors to detect touch, light, gravity and temperature. The reason why plants have developed pathways for electrical signal transmission most likely lies in the necessity to respond rapidly to environmental stress factors. Different environmental stimuli evoke specific responses in living cells. Living cells have the capacity to transmit signals to the other regions of the organism. In contrast to chemical signals such as hormones, electrical signals are able to rapidly transmit information over long distances.
Upon perception, electrical signals in plants can be propagated via the plasmodesmata to other cells of the symplast. Electrical coupling via the plasmodesmata was demonstrated in a variety of species, indicating that plasmodesmata are relays in the signaling network between plant cells.41 Low resistance connections extend between plant organs, and low resistance bridges are located throughout the whole plant. The sieve tube system appears to posses these qualities. The structures of the sieve tube members are unique and appear to be suitable for the transmission of electrical signals due to the relatively large, unoccluded sieve plate pores. The continuity of the plasma membrane appears to play a role in this process as well. Moreover, the low degree of electrical coupling in a lateral direction, caused by plasmodesmata at the interface between companion cells and phloem parenchyma cells, facilitates long distance signaling.
Despite the existence of great amounts of accumulated information concerning electric effects in plants, their physiological and electrochemical mechanisms remain poorly understood. Further investigation could provide information into the outlook of possible uses of these phenomena for improvement of agricultural technologies. This reason provides a significant basis to the importance of further profound investigations of electrical phenomena in plants. Green plants generate a wide spectrum of electric signals that arise in response to various external perturbations. These signals propagate for long distances along plant tissues and can cause remote effects in a plant.
All processes of life have been found to generate electric fields in every organism that has been examined with suitable and sufficiently sensitive measuring techniques. The conduction of electrochemical excitation must be regarded as one of the most universal properties of living organisms. Electrical signaling in living organism arose to address the need for the transmission of signals in response to an external influence from one part of a biological system to another. The study of the nature of regulatory relations of a plant organism with the environment is the investigation into a bioelectrochemical phenomenon providing insight into the growth and development of plants. According to modern measurements in real time, the APs in green plants and animals have similar speeds of propagation and duration. The automatic measurements of the extracellular and intracellular electrical potential difference can be effectively used in plant electrophysiology to study the molecular mechanisms of ion transport, the influence of external stimuli on plants, and for investigating the bioelectrochemical aspects of the interaction between plants and other biological organisms. The use of new computerized methods provides opportunities for detection of ultra fast APs in green plants in real time.
Materials and Methods
A novel real-time experimental setup using an ultra-fast PXI data acquisition system was developed (see Fig. 1). All measurements were conducted in the laboratory at constant room temperature inside a Faraday cage mounted on a vibration-stabilized table. In order to estimate possible high frequency content of the responses evoked, a high performance National Instruments data acquisition system was used. High speed data acquisition of low-pass filtered signals was performed using microcomputers with simultaneous multifunction I/O plug-in data acquisition board NI-PXI-6115 or NI-PCI-6115 (National Instruments) interfaced through a NI SCB-68 shielded connector block to 0.1 mm thick nonpolarizable reversible Ag/AgCl electrodes. The results were reproduced on a workstation with data acquisition board NI 6052E DAQ with input impedance of 100 GW interfaced through a NI SC-2040 Simultaneous Sample and Hold. The system integrates standard low-pass anti-aliasing filters at one half of the sampling frequency.
Ag/AgCl electrodes were prepared from Teflon coated silver wire (A-M Systems, Inc.). Localized thermal stress was created by flame applied continuously for 1 seconds to a predetermined leaf using a utility lighter (BIC). Ag/AgCl electrodes were prepared from Teflon coated silver wire (A-M Systems, Inc.). Ag/AgCl electrodes were maintained at constant temperature because of their high temperature sensitivity.
The soybean seedlings (Glycine max (L.) Merrill) were used approximately 3 weeks after germination. Each plant had an average of 5 developed leaves. Plants were grown in clay pots with 0.5 L of sterilized potting soil in a plant growth chamber (Environmental Corporation) at 22°C. Plants were watered daily and exposed to a 12/12 hr light/dark photoperiod at 22°C. Humidity remained constant at 45–50%.
Acknowledgements
Supported by National Science Foundation grant DMR-0521611 and NASA grant NAG8 -1888.
Abbreviations
- AP
action potential
- R
resistance
- C
capacitance
- fs
the sampling frequency
- fmax
the maximum frequency of the sampled signal
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/5586
References
- 1.Goldsworthy A. The evolution of plant action potentials. J Theor Biol. 1983;103:645–648. [Google Scholar]
- 2.Ksenzhek OS, Volkov AG. Plant Energetics. San Diego: Academic Press; 1998. [Google Scholar]
- 3.Labady A, Thomas DJ, Shvetsova T, Volkov AG. Plant electrophysiology: Excitation waves and effects of CCCP on electrical signaling in soybean. Bioelectrochem. 2002;57:47–53. doi: 10.1016/s1567-5394(01)00175-x. [DOI] [PubMed] [Google Scholar]
- 4.Volkov AG. Green plants: Electrochemical interfaces. J Electroanal Chem. 2000;483:150–156. [Google Scholar]
- 5.Volkov AG, editor. Plant Electrophysiology. Berlin: Springer; 2006. [Google Scholar]
- 6.Volkov AG. Electrophysiology and phototropism. In: Balushka F, Manusco S, Volkman D, editors. Communication in Plants. Neuronal Aspects of Plant Life. Berlin: Springer; 2006. pp. 351–367. [Google Scholar]
- 7.Volkov AG, Mwesigwa J. Electrochemistry of soybean: Effects of uncouplers, pollutants, and pesticides. J Electroanal Chem. 2001;496:153–157. [Google Scholar]
- 8.Volkov AG, Adesina T, Jovanov E. Closing of Venus flytrap by electrical stimulation of motor cells. Plant Signal Behavior. 2007;2:139–145. doi: 10.4161/psb.2.3.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volkov AG, Brown CL. Electrochemistry of plant life. In: Volkov AG, editor. Plant Electrophysiology. Berlin: Springer; 2006. pp. 437–459. [Google Scholar]
- 10.Bertholon M. De l'electricite des vegetaux: Ouvrage dans lequel on traite de l'electricite de l'atmosphere sur les plantes, de ses effets sur leconomie des vegetaux, de leurs vertus medico. Paris: PF Didotjeune; 1783. (Fre). [Google Scholar]
- 11.Bose JC. Transmission of stimuli in plants. Nature. 1925;115:457. [Google Scholar]
- 12.Burdon-Sanderson J. On the electromotive properties of the leaf of Dionaea in the excited and unexcited states. Phil Trans Royal Soc London. 1882;173:1–55. [Google Scholar]
- 13.Du Bois-Reymond E. Untersuchungen über thierishe elektrizität. Berlin: G Reiner; 1848. [Google Scholar]
- 14.Pickard BG. Action potentials in higher plants. Bot Reviews. 1973;38:172–201. [Google Scholar]
- 15.Favre P, Greppin H, Agosti RD. Repetitive action potentials induced in Arabidopsis thaliana leaves by wounding and potassium chloride application. Plant Physiol Biochem. 2001;39:961–969. [Google Scholar]
- 16.Malone M. Rapid, long-distance signal transmission in higher plants. Advances Botanical Research. 1996;22:163–228. [Google Scholar]
- 17.Sibaoka T. Physiology of rapid movements in higher plants. Annu Rev Plant Physiol. 1969;20:165–184. [Google Scholar]
- 18.Wildon DC, Thain JF, Minchin PEH, Gubb IR, Reilly AJ, Skipper YD, Doherty HM, O'Donnell PJ, Bowles DJ. Electric signaling and systemic proteinase inhibitor induction in the wounded plant. Nature. 1992;360:62–65. [Google Scholar]
- 19.Baker DA, Milburn JA. Appendix Table X: speed of assimilate transport in the phloem of plant species analyzed by different techniques. In: Baker DA, Milburn JA, editors. Transport of Photoassimilates. Harlow: Longman; 1989. pp. 354–355. [Google Scholar]
- 20.Passiora JB. The effect of root geometry on the yield of wheat growing on stored water. Australian J Agric Res. 1972;23:745–752. [Google Scholar]
- 21.Sacks J. Lectures on the Physiology of Plants. Oxford: Clarington Press; 1887. [Google Scholar]
- 22.Davies E. Action potentials as multifunctional signals in plants: A unifying hypothesis to explain apparently disparate wound responses. Plant Cell Environm. 1987;10:623–631. [Google Scholar]
- 23.Davies E. New functions for electrical signals in plants. New Phytologist. 2004;161:607–610. doi: 10.1111/j.1469-8137.2003.01018.x. [DOI] [PubMed] [Google Scholar]
- 24.Vian A, Davies E. Two different wound signals evoke very rapid, systemic CMBP transcript accumulation in tomato. Plant Signal Behavior. 2006;1:261–264. doi: 10.4161/psb.1.5.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burke JJ, Orzech KA. The heat-shock response in higher plants: A biochemical model. Plant Cell Environm. 1988;11:441–444. [Google Scholar]
- 26.Minorsky PV. Temperature sensing by plants: A review and hypothesis. Plant Cell Environm. 1989;12:119–135. [Google Scholar]
- 27.Malone M, Stankovic B. Surface potentials and hydraulic signals in wheat leaves following localized wounding by heat. Plant Cell Environm. 1991;14:431–436. [Google Scholar]
- 28.Lin CY, Roberts JK, Key JL. Acquisition of thermotolerance in soybean seedlings. Plant Physiol. 1984;74:152–160. doi: 10.1104/pp.74.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fender SE, O'Connell MA. Expression of the heat shock response in a tomato interspecific hybrid is not intermediate between the two parent responses. Plant Physiol. 1990;93:1140–1146. doi: 10.1104/pp.93.3.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhodes JD, Thain JF, Wildon DC. The pathway for systemic electrical signal conduction in the wounded tomato plant. Planta. 1996;200:50–57. [Google Scholar]
- 31.Stankovic B, Davies E. Both action potentials and variation potentials induce proteinase inhibitor gene expression in tomato. FEBS Lett. 1996;390:275–279. doi: 10.1016/0014-5793(96)00672-2. [DOI] [PubMed] [Google Scholar]
- 32.Hlavácková V, Krchnák P, Nauš J, Novák O, Špundová M, Strnad M. Electrical and chemical signals involved in short-term systemic photosynthetic responses of tobacco plants to local burning. Planta. 2006;225:235–244. doi: 10.1007/s00425-006-0325-x. [DOI] [PubMed] [Google Scholar]
- 33.Sinyukhin AM, Gorchakov VV. Action potentials of higher plants not possessing motor activity. Biophysics. 1966;11:966–974. [Google Scholar]
- 34.Pyatygin SS, Opritov VA. Effect of temperature on the generation of action potentials by the excitable cells of a higher plant. Biophysics. 1990;35:451–457. [Google Scholar]
- 35.Pyatygin SS, Opritov VA, Krauz VO, Polovinkin AV. Increase in cold resistance of electrogenesis as a basis for adaptive repolarization in higher plant cells during chilling. Russ J Plant Physiol. 1996;43:223–227. [Google Scholar]
- 36.Fromm J, Lautner S. electrical signals and their physiological significance in plants. Plant Cell Environm. 2007;30:249–257. doi: 10.1111/j.1365-3040.2006.01614.x. [DOI] [PubMed] [Google Scholar]
- 37.Koziolek C, Grams TEE, Schreiber U, Matysek R, Fromm J. Transient knockout of photosynthesis mediated by electrical signals. New Phytologist. 2003;161:715–722. doi: 10.1111/j.1469-8137.2004.00985.x. [DOI] [PubMed] [Google Scholar]
- 38.Fromm J, Hajirezaei M, Wilke I. The biochemical response of electrical signaling in the reproductive system of Hibiscus plants. Plant Physiol. 1995;109:375–384. doi: 10.1104/pp.109.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volkov AG, Lang RD, Volkova-Gugeshashvili MI. Electrical signaling in Aloe vera induced by localized thermal stress. Bioelectrochem. 2007;71:192–197. doi: 10.1016/j.bioelechem.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Roshchina VV. Neurotransmitters in Plant Life. Enfield: Science Publishers; 2001. [Google Scholar]
- 41.Shimmen T. Electrophysiology in mechanosensing and wounding response. In: Volkov AG, editor. Plant Electrophysiology. Berlin: Springer; 2006. pp. 319–339. [Google Scholar]