Abstract
Using a live-cell imaging approach to study individual micro-tubules, we have compared microtubule behavior between net-like and aligned cortical arrays. In contrast to previous studies, a steep angled collision between the growing end of a microtubule and a preexisting microtubule was found to favor crossover. Frequencies of microtubule crossovers, bundling and catastrophes are similar regardless of whether the cell exhibited a net-like or aligned microtubule array. In the predominantly aligned array of petiole cells, severing occurs at the sites of microtubule crossovers and serves to remove unaligned microtubules and to increase microtubule density. Severing was observed to be rare in net-like arrays. Microtubule severing is carried out by the katanin enzyme. In this addendum, we present new insights into the possible mechanism of crossing over and preliminary data looking at organization of the array in a katanin mutant.
Key words: cortical array, collision, catastrophe, severing, katanin, petiole, pavement, cotyledons, YFP
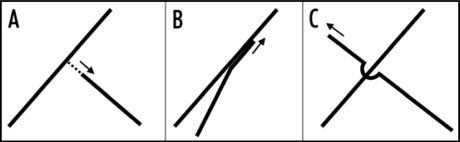
In plants, proper cell expansion requires the transformation of the cortical microtubule array from a net-like configuration to an aligned and predominantly transverse configuration. The mechanisms underlying this transition have been, until recently, poorly understood. Previous observations in tobacco BY2 cells suggested that the outcome of a collision between a growing microtubule plus end and an existing microtubule determined the configuration of the array.1 In microtubule arrays of BY2 cells, steep angled collisions favor rapid depolymerization, termed a catastrophe, whereas shallow angled collisions promote bundling. These different collision outcomes are depicted in figures 1a and 1b. By applying these experimental observations to a computer model of BY2 microtubules, a disordered array could be seen to gradually transform into an aligned array.
The cotyledons of Arabidopsis seedlings are well suited for determining how interactions between individual microtubules affect the configuration of the whole array.2 Pavement cells exhibit net-like arrays whereas petiole cells exhibit aligned arrays. By using a yellow fluorescent protein-based microtubule reporter that exclusively labels arrays of the outermost cell layer of the cotyledon, the behavior of microtubules were examined in pavement and petiole cells and compared with the data from the BY2 study.1
In both Arabidopsis and BY2 cells, shallow angle collisions promote bundling and we expected catastrophe frequency from steep-angled collisions to be similar in BY2 cells and Arabidopsis. However, although steep angled collisions at the growing ends of microtubules in BY2 cells exhibit a catastrophe frequency of 60%, this is much reduced in Arabidopsis. The net-like arrays of pavement cells and the aligned arrays of petiole cells exhibit catastrophe frequencies of 24% and 9%, respectively.2 The data suggests that in Arabidopsis the transition from net-like to aligned microtubule arrangement is accompanied by a decrease in the catastrophe frequency, with most steep-angled collisions resulting in a crossover event (Fig. 1C). A crossover event occurs when the growing leading end of a microtubule overcomes an obstacle in the form of an existing microtubule. The mechanism that allows a growing microtubule end to form a crossover is not fully understood, however, the end of the growing face of the microtubule is likely to be in contact with the obstruction because crossovers are accompanied by a reduction in the rate of microtubule growth.2 These data suggest that, for Arabidopsis, growing ends are somehow stabilized and resist the catastrophes observed in BY2 cells. In Xenopus, XMAP215 increases microtubule growth rates and reduces the catastrophe frequency in egg extracts during interphase3 as well as increasing crossovers similar to those seen in Arabidopsis (http://www.mpi-cbg.de/downloads/movie/interRT.mov). Consistent with this observation, depletion of XMAP215 in Xenopus extracts results in an increase in the microtubule catastrophe frequency. A temperature sensitive mutant allele of mor1, the Arabidopsis homolog of XMAP215 results in shorter cortical microtubules and loss of parallel alignment.4 MOR1 is therefore a strong candidate for a stabilizing factor during microtubule crossover.
Figure 1.
Schematic representations of collision outcomes (A) catastrophe (B) bundling (C) crossover.
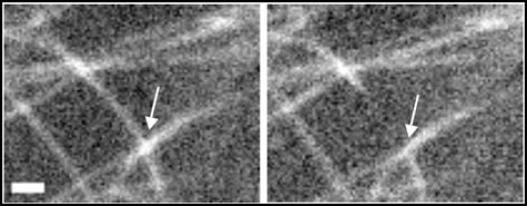
Both the net-like arrays of pavement cells and the aligned arrays of petiole cells exhibit high levels of microtubule crossover, suggesting that some other mechanism contributes to array alignment. A detailed analysis of microtubule dynamics in the aligned arrays of the petiole cells revealed a single behavior that was markedly increased compared to the pavement cells—severing at the sites of microtubule crossover.2 A severing event from an aligning array is shown in Figure 2. Severing results in removal of the posterior portion of the unaligned microtubule whilst the anterior portion can grow in an arc and bundle with the rest of the array. The posterior portion either depolymerizes completely or forms a new microtubule that grows in a new direction in an attempt to align with the rest of the array. This means that severing at crossovers has two roles, removing those microtubules that are not aligned with the rest of the array and increasing microtubule density. This results in a densely packed, aligned array as seen in petiole cells.
Figure 2.
A microtubule crossover before (left panel) and immediately after (right panel) severing. Site of crossover is shown by an arrow. Bar = 1 µm.
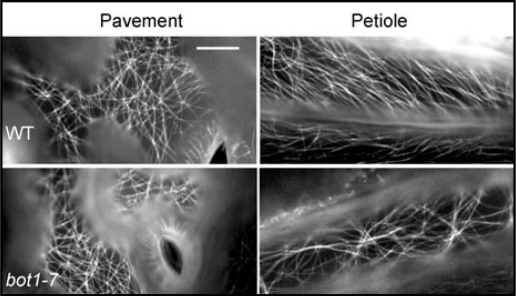
In higher eukaryotes, severing is carried out by the enzyme katanin.5 In Arabidopsis, several katanin mutants have been described and exhibit phenotypes consistent with a defect in cell expansion.6–9 In one such mutant, called botero1, roots fail to make the transition from a net-like array in the division zone to the aligned array in the elongation zone. Historically, the relationship between katanin function and array alignment has been hard to interpret because katanin function has only been associated with severing at the sites of microtubule nucleation. According to our data, alignment is dependent upon severing at crossovers, therefore, in a katanin mutant, petiole cells are predicted to contain only net-like arrays. Figure 3 compares microtubule arrays in pavement and petiole cells between wild-type Arabidopsis and the botero1–7 mutant. The cortical arrays of pavement cells are very similar between WT and mutant suggesting katanin function is not important for the generation of a net-like array. However, in petiole cells of botero1–7 the microtubules fail to adopt the aligned configuration observed in WT. This failure of array alignment in the petiole cells has been observed previously for another katanin mutant, fra2.8
Figure 3.
Comparison of microtubule arrays between WT (ecotype Ws) and botero1–7 plants. Note the alignment defect in petioles of botero1–7. Bar = 10 µm.
To determine the relative effects of severing and other behaviors of microtubules upon array organization, accurate modeling of micro- tubules will be essential. Work is currently underway to mimic, in detail, microtubule dynamics using advanced computer modeling techniques (Marshall R, Department of Physics and Astronomy, University of Manchester, personal communication). This modeling will include user changeable parameters such as severing frequency, catastrophe frequency and the outcome of a catastrophe. Comparing the models with biological systems will increase our understanding of the importance of the various behaviours in array organisation.
Acknowledgements
The authors thank Peter Etchells for assistance with proofreading this addendum. The work described in this addendum was supported by the Biotechnology and Biological Sciences Research Council, grant no. 34/C19282.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/5140
References
- 1.Dixit R, Cyr R. Encounters between dynamic cortical microtubules promote ordering of the cortical array through angle-dependent modifications of microtubule behavior. Plant Cell. 2004;16:3274–3284. doi: 10.1105/tpc.104.026930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wightman R, Turner SR. Severing at sites of microtubule crossover contributes to microtubule alignment in cortical arrays. Plant J. 2007 doi: 10.1111/j.1365-313X.2007.03271.x. In press. [DOI] [PubMed] [Google Scholar]
- 3.Tournebize R, Popov A, Kinoshita K, Ashford AJ, Rybina S, Pozniakovsky A, Mayer TU, Walczak CE, Karsenti E, Hyman AA. Control of microtubule dynamics by the antagonistic activities of XMAP215 and XKCM1 in Xenopus egg extracts. Nat Cell Biol. 2000;2:13–19. doi: 10.1038/71330. [DOI] [PubMed] [Google Scholar]
- 4.Whittington AT, Vugrek O, Wei KJ, Hasenbein NG, Sugimoto K, Rashbrooke MC, Wasteneys GO. MOR1 is essential for organizing cortical microtubules in plants. Nature. 2001;411:610–613. doi: 10.1038/35079128. [DOI] [PubMed] [Google Scholar]
- 5.McNally FJ, Vale RD. Identification of katanin, an ATPase that severs and disassembles stable microtubules. Cell. 1993;75:419–429. doi: 10.1016/0092-8674(93)90377-3. [DOI] [PubMed] [Google Scholar]
- 6.Bichet A, Desnos T, Turner S, Grandjean O, Hofte H. BOTERO1 is required for normal orientation of cortical microtubules and anisotropic cell expansion in Arabidopsis. Plant J. 2001;25:137–148. doi: 10.1046/j.1365-313x.2001.00946.x. [DOI] [PubMed] [Google Scholar]
- 7.Bouquin T, Mattsson O, Naested H, Foster R, Mundy J. The Arabidopsis lue1 mutant defines a katanin p60 ortholog involved in hormonal control of microtubule orientation during cell growth. J Cell Sci. 2003;116:791–801. doi: 10.1242/jcs.00274. [DOI] [PubMed] [Google Scholar]
- 8.Burk DH, Liu B, Zhong R, Morrison WH, Ye ZH. A katanin-like protein regulates normal cell wall biosynthesis and cell elongation. Plant Cell. 2001;13:807–827. [PMC free article] [PubMed] [Google Scholar]
- 9.Webb M, Jouannic S, Foreman J, Linstead P, Dolan L. Cell specification in the Arabidopsis root epidermis requires the activity of ECTOPIC ROOT HAIR 3-a katanin p60 protein. Development. 2002;129:123–131. doi: 10.1242/dev.129.1.123. [DOI] [PubMed] [Google Scholar]