Abstract
The Arabidopsis class-1 KNOX genes STM, BP/KNAT1, KNAT2 and KNAT6 encode homeodomain transcriptional regulators important for shoot apical meristem (SAM) and carpel development. During vegetative growth, STM is required to establish and maintain the stem cell pool of the SAM, a function replaceable by ectopic expression of BP/KNAT1 but not of other class-1 KNOX genes. We recently demonstrated additional STM roles in the development of the central floral whorl and subsequent formation of carpels, a function replaceable by ectopic KNAT2 expression. However, STM is normally required for BP/KNAT1 and KNAT2 expression, explaining why it is essential for both SAM and carpel development. We propose therefore that STM provides the critical KNOX function in these processes and that the SAM- and carpel-promoting activities of STM are redundantly duplicated in BP/KNAT1 and KNAT2 respectively. Here we show that ectopic KNAT2 expression can restore carpel development to stm mutants, but fails to restore proper development of the central floral whorl, which requires a function analogous to the SAM-promoting activities of STM and BP/KNAT1. Similarly, we show that ectopic KNAT2 expression does not restore normal meristem organisation to the SAM. We propose a model for discrete and overlapping class-1 KNOX gene function in Arabidopsis.
Key words: KNOX gene, SHOOT MERISTEMLESS, KNAT, meristem, carpel, stem cell, KNOTTED
The KNOTTED1-like homeobox (KNOX) genes are found in all higher plant species and encode homeodomain transcription factors similar to those that regulate development in animals.1 The class-1 KNOX gene subfamily in Arabidopsis comprises SHOOT MERISTEMLESS (STM), BREVIPEDICELLUS/KNAT1/ (BP/KNAT1), KNAT2 and KNAT6, all of which are expressed in the shoot apical meristem (SAM) but not in lateral organs, where ectopic expression results in inhibited cell expansion and differentiation during leaf development.2–6
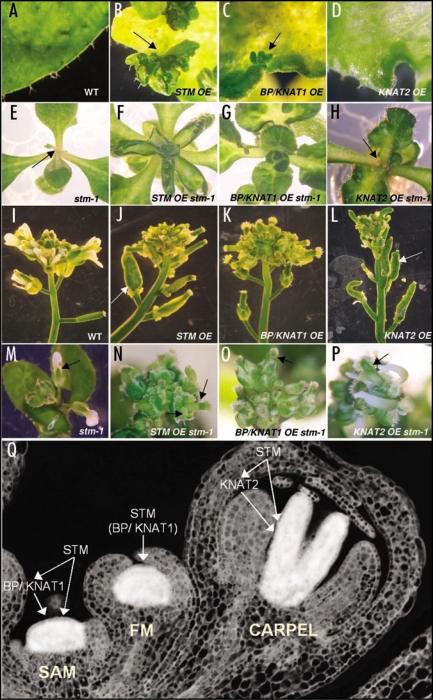
Loss-of-function mutations in STM perturb or abolish the maintenance of the SAM because STM is required to maintain the stem cell pool, whereas mutation of other class-1 KNOX genes has no effect on SAM development (Fig. 1E).7–9 Conversely, ectopic expression of STM leads to the formation of ectopic SAMs on the adaxial leaf surface, indicating that STM is sufficient to activate de novo SAM formation (Fig. 1B).3,4 The closely related gene BP/KNAT1 can also activate ectopic SAM formation when overexpressed, and can substitute for STM in SAM development if de-repressed or induced artificially when STM function is compromised (Fig. 1C and G).10–12 However, loss of BP/KNAT1 function does not affect SAM development but instead results in reduced growth of floral internodes, pedicels and the style during reproductive growth, consistent with its expression in these tissues/organs.13,14
Figure 1.
Discrete and overlapping roles of the Arabidopsis class-1 KNOX genes. (A–D) Overexpression of KNOX genes in leaves in the WT (Ler) background. Induced KNOX gene expression levels were similar in the various lines. (A) WT leaf margin. (B) STM overexpressor (STM OE) leaf. Ectopic shoots are formed (arrow). (C) BP/KNAT1 overexpressor (BP/KNAT1 OE) leaf. Ectopic shoots are formed (arrow). (D) KNAT2 overexpressor (KNAT2 OE) leaf. No ectopic shoots are formed. (E–H) Overexpression of KNOX genes in stm-1 shoot apices. (E) stm-1 apex. Arrested shoot apex after the formation of a few adventitious leaves (arrow), as is often seen following 2–3 weeks growth of stm-1 plants in vitro. (F) STM OE stm-1 apex. Normal shoot activity is restored. (G) BP/KNAT1 OE stm-1 apex. Normal shoot activity is restored. (H) KNAT2 OE stm-1 apex. Shoot activity is not restored (arrow). (I–L) Overexpression of KNOX genes in WT (Ler) inflorescences. (I) WT (Ler) inflorescence. (J) STM OE, (K) BP/KNAT1 OE and (L) KNAT2 OE inflorescences. Ovule to carpel transformation5,16 causes swelling of primary gynoecium in STM OE (J) and KNAT2 OE flowers (L) only (arrows). (M–P) Overexpression of KNOX genes in stm-1 inflorescences. (M) stm-1 inflorescence. Terminal flowers lack central carpels/ gynoecium (arrow). (N) STM OE stm-1 inflorescence. Gynoecium and ectopic carpels develop (arrows). (O) KNAT1 OE stm-1. Gynoecium development is restored (arrow) but no ectopic carpels develop. (P) KNAT2 OE stm-1. Carpels form, but not the central gynoecium (arrow). (Q) Model for Arabidopsis class-1 KNOX gene function in the SAM, FM and carpel. STM is required for BP/KNAT1 expression in the SAM (left), and both genes can promote SAM formation, although BP/KNAT1 is not normally essential for SAM development. In the FM (centre), STM is required to maintain the central stem cells, and BP/KNAT1 can also perform this function when ectopically expressed, although it is not normally expressed in this domain. STM and KNAT2 promote carpel development (right). STM is normally required for KNAT2 expression, but not when the FM stem cells are maintained by ectopic BP/KNAT1 expression. KNAT2 cannot maintain stem cells in the FM, and is not essential for carpel development.12,15
KNOX genes are also involved in reproductive development. Ectopic expression of KNAT2 (but not BP/KNAT1 or KNAT6) during flowering induces the formation of ectopic carpels and the homeotic conversion of ovules to carpels,5 although KNAT2 is not essential for carpel formation in normal development because mutation of KNAT2 confers no discernable phenotype.12,15 Interestingly, we have recently shown that STM induces similar phenotypic changes to KNAT2 when ectopically expressed in flowers.16 Moreover, using inducible loss-of-function, we demonstrated that STM is required for the development of the central floral whorl, where it transiently maintains the central floral stem cells and subsequently directs the formation of carpels and the placental tissues from which ovules arise. These phenotypic changes are consistent with the expression of STM (and KNAT2) in the centre of the developing flower and in carpel placentae.7,17 Thus, STM has an essential role in floral meristem (FM) and carpel development in addition to performing a critical function in the SAM.
We assessed the different functions of the class-1 KNOX genes in shoot and flower development using inducible ectopic expression in the wild-type (Landsberg erecta; Ler) and stm-1 mutant backgrounds (Fig. 1). Induced expression of BP/KNAT1 fully restored SAM function to stm mutants, though the reproductive SAM was not as robustly maintained as the vegetative SAM (Fig. 1G). Interestingly, development of the two fused carpels of the gynoecium was variably restored in stm-1 flowers overexpressing BP/KNAT1, with many anatomically normal gynoecia formed (Fig. 1O). However, no ectopic carpels or carpelloid ovules were observed, whereas these are seen with ectopic STM and KNAT2 expression (Fig. 1J, L, N and P). Conversely, ectopic expression of KNAT2 failed to promote ectopic SAM formation, restore primary SAM function or proper central whorl formation in stm mutant flowers (Fig. 1 D, H and P). Although fused gynoecia were rarely formed, many ectopic carpels were initiated in stm-1 flowers overexpressing KNAT2. These observations support the conclusion that BP/KNAT1 has SAM-specific roles, whereas KNAT2 has carpel specific roles, and demonstrate that these genes can functionally substitute for STM in these respective locations. Since STM is required for expression of both KNAT1/BP and KNAT2 in vegetative and reproductive growth (Scofield and Murray, unpublished data),16 the functional redundancy of BP/KNAT1 and KNAT2 is not apparent unless their expression is manipulated artificially.
It is intriguing that BP/KNAT1 is able to restore gynoecium development to stm mutant flowers, given that it is not normally expressed in the centre of the FM and its ectopic expression does not result in carpelloid features, and at first sight this appears to contradict the model of specific roles. However, the roles of STM in floral development are in fact two fold. Its first function is to sustain a stem cell population in the central zone of the floral meristem, and this function, directly analogous to its maintenance role for the stem cell population of the SAM, can be compensated by ectopic BP/KNAT1 in stm flowers which maintains the central zone of floral stem cells during the initial stages of floral meristem development. This creates a domain in which KNAT2 expression can occur and induce carpel formation. In agreement with this hypothesis, we detected normal levels of KNAT2 expression in stm mutant flowers overexpressing BP/KNAT1 (data not shown), indicating that KNAT2 is probably functional.
We propose a general model for class-1 KNOX gene function in Arabidopsis (Fig. 1Q). STM is essential for SAM formation and maintenance during both vegetative and reproductive growth, as well as for central floral whorl establishment and carpel formation during flowering. The vegetative SAM is maintained because STM inhibits the cellular differentiation normally associated with organogenesis and permits the WUS-CLAVATA feedback loop to maintain the central stem cells.18,19 This function can also be performed by BP/KNAT1 when de-repressed or ectopically expressed (Fig. 1G).11–12 In floral meristems, the stem cells must be transiently maintained in order to generate the central whorl of carpels. STM is critical not only for this initial maintenance of the stem cells, a function that as show here can also be performed by BP/KNAT1, but also for the subsequent formation of carpels and carpel placentae following the termination of stem cell maintenance by AGAMOUS.20,21 This second function can also be performed by KNAT2. Since STM is required for the expression of both BP/KNAT1 and KNAT2, the redundant functions of these genes are not performed when STM function is lost, resulting in the stm phenotype. We propose that KNOX genes, and STM in particular, are key conserved factors in several types of meristematic tissue in the shoot: those that generate vegetative organs and flowers (SAM), those that generate reproductive organs (FM central zone) and those that generate female gametes (carpel placentae). In addition, BP/KNAT1 plays roles in the growth of certain structures such as the floral stalk. Thus, our work shows that various different types of meristematic tissue share a common molecular regulatory mechanism. Interaction with growth phase- or tissue-specific factors, or accessibility to downstream target genes, likely determines the type of meristematic tissue produced by KNOX genes, and interaction with such factors is probably conserved for STM and BP/KNAT1 in the SAM and STM and KNAT2 in the carpel.
Acknowledgments
This work was funded by the ERA-PG Network BB/E024858/1 “Integrated analysis of plant stem cell function in growth and development”, the Cambridge Isaac Newton Trust, and the EU SY-STEM (Systems Biology of stem cell function in Arabidopsis thaliana) network (contract no. MRTN-CT-2004-005336).
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/5194
References
- 1.Scofield S, Murray JAH. KNOX gene function in plant stem cell niches. Plant Mol Biol. 2006;60:929–946. doi: 10.1007/s11103-005-4478-y. [DOI] [PubMed] [Google Scholar]
- 2.Lincoln C, Long J, Yamaguchi J, Serikawa K, Hake S. A knotted1-like homeobox gene in Arabidopsis is expressed in the vegetative meristem and dramatically alters leaf morphology when overexpressed in transgenic plants. Plant Cell. 1994;6:1859–1876. doi: 10.1105/tpc.6.12.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brand U, Grunewald M, Hobe M, Simon R. Regulation of CLV3 expression by two homeobox genes in Arabidopsis. Plant Physiol. 2002;129:565–575. doi: 10.1104/pp.001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lenhard M, Jurgens G, Laux T. The WUSCHEL and SHOOTMERISTEMLESS genes fulfil complementary roles in Arabidopsis shoot meristem regulation. Development. 2002;129:3195–3206. doi: 10.1242/dev.129.13.3195. [DOI] [PubMed] [Google Scholar]
- 5.Pautot V, Dockx J, Hamant O, Kronenberger J, Grandjean O, Jublot D, Traas J. KNAT2: Evidence for a link between knotted-like genes and carpel development. Plant Cell. 2001;13:1719–1734. doi: 10.1105/TPC.010184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dean G, Casson S, Lindsey K. KNAT6 gene of Arabidopsis is expressed in roots and is required for correct lateral root formation. Plant Mol Biol. 2004;54:71–84. doi: 10.1023/B:PLAN.0000028772.22892.2d. [DOI] [PubMed] [Google Scholar]
- 7.Long JA, Moan EI, Medford JI, Barton MK. A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature. 1996;379:66–69. doi: 10.1038/379066a0. [DOI] [PubMed] [Google Scholar]
- 8.Clark SE, Jacobsen SE, Levin JZ, Meyerowitz EM. The CLAVATA and SHOOT MERISTEMLESS loci competitively regulate meristem activity in Arabidopsis. Development. 1996;122:1567–1575. doi: 10.1242/dev.122.5.1567. [DOI] [PubMed] [Google Scholar]
- 9.Endrizzi K, Moussian B, Haecker A, Levin JZ, Laux T. The SHOOT MERISTEMLESS gene is required for maintenance of undifferentiated cells in Arabidopsis shoot and floral meristems and acts at a different regulatory level than the meristem genes WUSCHEL and ZWILLE. Plant J. 1996;10:967–979. doi: 10.1046/j.1365-313x.1996.10060967.x. [DOI] [PubMed] [Google Scholar]
- 10.Chuck G, Lincoln C, Hake S. KNAT1 induces lobed leaves with ectopic meristems when overexpressed in Arabidopsis. Plant Cell. 1996;8:1277–1289. doi: 10.1105/tpc.8.8.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrne ME, Barley R, Curtis M, Arroyo JM, Dunham M, Hudson A, Martienssen RA. Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature. 2000;408:967–971. doi: 10.1038/35050091. [DOI] [PubMed] [Google Scholar]
- 12.Byrne ME, Simorowski J, Martienssen RA. ASYMMETRIC LEAVES1 reveals knox gene redundancy in Arabidopsis. Development. 2002;129:1957–1965. doi: 10.1242/dev.129.8.1957. [DOI] [PubMed] [Google Scholar]
- 13.Douglas SJ, Chuck G, Dengler RE, Pelecanda L, Riggs CD. KNAT1 and ERECTA regulate inflorescence architecture in Arabidopsis. Plant Cell. 2002;14:547–558. doi: 10.1105/tpc.010391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venglat SP, Dumonceaux T, Rozwadowski K, Parnell L, Babic V, Keller W, Martienssen R, Selvaraj G, Datla R. The homeobox gene BREVIPEDICELLUS is a key regulator of inflorescence architecture in Arabidopsis. Proc Natl Acad Sci USA. 2002;99:4730–4735. doi: 10.1073/pnas.072626099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belles-Boix E, Hamant O, Witiak SM, Morin H, Traas J, Pautot V. KNAT6: An Arabidopsis homeobox gene involved in meristem activity and organ separation. Plant Cell. 2006;18:1900–1907. doi: 10.1105/tpc.106.041988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scofield S, Dewitte W, Murray JAH. The KNOX gene SHOOT MERISTEMLESS is required for the development of reproductive meristematic tissues in Arabidopsis. Plant J. 2007;50:767–781. doi: 10.1111/j.1365-313X.2007.03095.x. [DOI] [PubMed] [Google Scholar]
- 17.Long J, Barton MK. Initiation of axillary and floral meristems in Arabidopsis. Dev Biol. 2000;218:341–353. doi: 10.1006/dbio.1999.9572. [DOI] [PubMed] [Google Scholar]
- 18.Brand U, Fletcher JC, Hobe M, Meyerowitz EM, Simon R. Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science. 2000;289:617–619. doi: 10.1126/science.289.5479.617. [DOI] [PubMed] [Google Scholar]
- 19.Schoof H, Lenhard M, Haecker A, Mayer KF, Jurgens G, Laux T. The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell. 2000;100:635–644. doi: 10.1016/s0092-8674(00)80700-x. [DOI] [PubMed] [Google Scholar]
- 20.Lenhard M, Bohnert A, Jurgens G, Laux T. Termination of stem cell maintenance in Arabidopsis floral meristems by interactions between WUSCHEL and AGAMOUS. Cell. 2001;105:805–814. doi: 10.1016/s0092-8674(01)00390-7. [DOI] [PubMed] [Google Scholar]
- 21.Lohmann JU, Hong RL, Hobe M, Busch MA, Parcy F, Simon R, Weigel D. A molecular link between stem cell regulation and floral patterning in Arabidopsis. Cell. 2001;105:793–803. doi: 10.1016/s0092-8674(01)00384-1. [DOI] [PubMed] [Google Scholar]