Abstract
Accumulating evidence indicates that plant growth promoting rhizobacteria (PGPR) influence plant growth and development by the production of phytohormones such as auxins, gibberellins, and cytokinins. Little is known on the genetic basis and signal transduction components that mediate the beneficial effects of PGPRs in plants. We recently reported the identification of a Bacillus megaterium strain that promoted growth of A. thaliana and P. vulgaris seedlings. In this addendum, the role of cytokinin signaling in mediating the plant responses to bacterial inoculation was investigated using A. thaliana mutants lacking one, two or three of the putative cytokinin receptors CRE1, AHK2 and AHK3, and RPN12 a gene involved in cytokinin signaling. We show that plant growth promotion by B. megaterium is reduced in AHK2-2 single and double mutant combinations and in RPN12. Furthermore, the triple cytokinin-receptor CRE1-12/AHK2-2/AHK3-3 knockout was insensitive to inoculation in terms of growth promotion and root developmental responses. Our results indicate that cytokinin receptors play a complimentary role in plant growth promotion by B. megaterium.
Key words: Arabidopsis, plant growth stimulation, root development, rhizobacteria
Introduction
A number of rhizobacterial species associate with plants to increase bacterial fitness and plant growth. Plant growth promoting rhizobacteria (PGPR) are free living, rhizosphere-inhabiting bacteria that have a positive influence on plant growth and development.1,2 These microorganisms, which belong to diverse genera such as Pseudomonas, Azospirillum and Bacillus, have been recognized from a wide range of plant species, such as barley, rice canola, bean and Arabidopsis.3–5 The contribution of PGPR to plant growth can be exerted by mechanisms that include competition with deleterious microorganisms,6 the activation of plant defense responses7,8 and secretion of plant growth-regulating substances such as auxins, cytokinins and bacterial volatiles.9–12 Phytohormones are involved in the control of growth and in almost every important developmental process in plants. Bacterial secretion of phytohormones can impact root architecture by overproduction of root hairs and lateral roots and subsequently increase nutrient and water uptake, thus contributing to growth.2
To elucidate the signaling mechanisms by which PGPR promote growth and modify plant development, we used Arabidopsis thaliana as a model plant to identify bacterial strains with plant growth promoting activity. Our research identified a new strain of Bacillus megaterium (UMCV1), which promoted growth of Arabidopsis thaliana and Phaseolus vulgaris plants in vitro and in soil. Inoculation with B. megaterium affected the root system in A. thaliana WT plants in a way that suggested the effects mediated by phytohormones, including an inhibition in primary root growth followed by an increase in lateral root number, lateral root growth and root hair length.13 The effects of bacterial inoculation on seedling growth were found to be independent of auxin- and ethylene- signaling as revealed by increased biomass production and lateral root growth stimulation of auxin-resistant mutants aux1-7, axr4-1 and eir1-1 and ethylene-response mutants etr1-1 and ein2-1. Moreover, B. megaterium inoculation failed to enhance the expression of the DR5: GUS auxin-inducible gene marker indicating that this bacteria might not produce auxins.13 These observations indicate that the signaling pathways involved in growth stimulation in response to inoculation with B. megaterium are likely to be identified in plants.
A Role for Cytokin Signaling in Plant Growth Stimulation by PGPR
Cytokinins are a class of phytohormones produced by plants and microorganisms.14 Their production by plant-associated bacteria has been well documented.15,16 Thus, it can be expected that plant inoculation with bacterial species capable of producing cytokinins may increase the level of cytokinins in root tissues. In turn, this may have an impact on plant growth. In this study, the involvement of cytokinin signaling in mediating the growth and developmental responses of plants to B. megaterium inoculation was tested. Three A. thaliana cytokinin receptors, CRE1, AHK2 and AHK3 have been identified (described in refs. 17–20). Single and double mutants of the CRE1, AHK2 and AHK3 genes have normal root development. However, the cre1-12/ahk2-2/ahh3-3 triple mutant showed retarded growth of the primary root and arrested shoot development.17,18 To investigate whether B. megaterium inoculation could involve the cytokinin-signaling pathway, we evaluated the effects of bacterial inoculation at 5 cm from the primary root meristem in 5-day WT plants (Col-0 and C-24) and cre1-12, ahk2-2tk, ahk3-3, cre1-12/ahk2-2tk, cre1-12/ahk3-3, and ahk2-2tk/ahk3-3 mutant seedlings that were grown in Murashige and Skoog (MS) 0.2x nutrient medium.
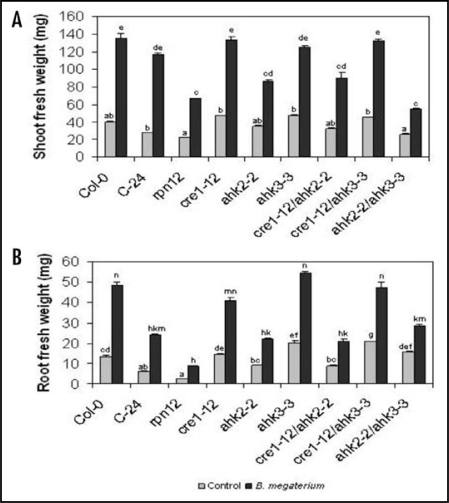
After 6 days of growth in the presence of B. megaterium the shoot and root biomass were quantified. As shown in Figure 1, bacterial inoculation caused a 3-fold increase in shoot and root fresh weight in WT plants from the Col-0 and C-24 ecotypes. In contrast, the growth promoting effects of inoculation were reduced in rpn12a-1 (C-24 background), which is defective in a subunit of the Arabidopsis 26S proteasome important for numerous cytokinin regulated growth responses,21 and in single and double mutant combinations involving the AHK2-2 cytokinin receptor (Fig. 1A and B). Next we tested the effects of B. megaterium inoculation on growth and root development in the triple cytokinin receptor mutant cre1-12/ahk2-2tk/ahk3-3. The triple mutant is obtained from a cre1-12/cre1-12 ahk2-2tk/ahk2-2tk ahk3-3/AHK3 heterozigous population. In agar medium homozygous cre1-12/ahk2-2tk/ahk3-3 triple mutants develop short primary roots and can be easily distinguished from cre1-12/cre1-12 ahk2-2tk/ahk2-2tk ahk3-3/AHK3 heterozygous plants. To select for the triple mutants, a pool of seeds produced by cre1-12/cre1-12 ahk2-2tk/ahk2-2tk ahk3-3/AHK3 plant were sterilized and sown on agar plates. After 10 days, seedlings with short primary roots were selected and transferred to plates with MS 0.2x fresh medium.
Figure 1.
Effects of Bacillus megaterium on growth of wild-type lines of Arabidopsis (Col-0 and C-24 ecotypes), and on cytokinin-signaling mutants. Plant material was harvested 6 days after bacterial inoculation at 5 cm from the root tip. Shoots and roots were excised at the root-shoot junction and the fresh weight was determined on an analytical balance. (A) Shoot fresh weight. (B) Root fresh weight. Values shown represent the mean of four groups of 20 seedlings ± standard deviation. Different letters are used to indicate means that differ significantly (p < 0.05).
At this time, bacterial inoculation was performed at 2 and 5 cm of distance from root tips and growth and development scored at a further 6-day period. In WT (Col-0) plants, bacterial inoculation stimulated growth and development. In particular, inoculated plants developed a robust root system with proliferating lateral roots (Fig. 2A and B). Growth was severely impaired in cre1-12/ahk2-2tk/ahk3-3 uninoculated triple mutants, giving rise to dwarf plants with small roots. In these plants, bacterial inoculation at 2 or 5 cm failed to stimulate growth and root development (Fig. 2C–E). Taken together, our results suggest that AHK2 and RPN12 play an important role in growth promotion by B. megaterium and that the three cytokinin receptors are required for normal growth and developmental responses to bacterial inoculation.
Figure 2.
Effect of Bacillus megaterium on growth and development of wild-type Arabidopsis (Col-0) and cre1-12/ahk2-2tk/ahk3-3 triple cytokinin receptor mutant. (A) Arabidopsis (Col-0) plants grown on the surface of agar plates with 0.2x Murashige and Skoog (MS) medium. (B) Arabidopsis plants that were inoculated with B. megaterium at a distance of 5 cm from the root tip 5 days after germination and grown for a further 6-day period. (C) cre1-12/ahk2-2tk/ahk3-3 seedlings uninoculated, (D) inoculated at 5 cm or (E) 2 cm from the primary root tip. Note the elicitation of growth by bacterial inoculation and the formation of branched root systems in WT plants. These effects were absent in the triple cytokinin receptor mutants. Photographs are representative individuals from four plates per treatment.
Although the signaling network between plants and rhizobacteria has been extensively studied over the past 20 years, a very few molecular components involved in the interaction between the bacteria and the plant had been reported until recently.2 Our work extends this previous knowledge by showing that growth stimulation by B. megaterium requires an intact cytokinin-signaling pathway in A. thaliana. It is interesting that many PGPR from the rhizosphere can produce cytokinins that exert a pronounced growth stimulatory effect in different crop plants.9,11 This effect can be mediated by different cytokinin receptor homologs.
Acknowledgements
We are thankful to Drs. Tatsuo Kakimoto and Richard D. Vierstra for kindly providing us with A. thaliana mutant seeds.
Footnotes
Previously published online as a Plant Signaling & behavior E-publication: www.landesbioscience.com/journals/psb/article/5204
References
- 1.Bloemberg GV, Lugtenberg BJJ. Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr Op Plant Biol. 2001;4:343–350. doi: 10.1016/s1369-5266(00)00183-7. [DOI] [PubMed] [Google Scholar]
- 2.Persello-Cartieaux F, Nussaume L, Robaglia C. Tales from the underground: Molecular plant-rhizobacteria interactions. Plant Cell Environ. 2003;26:189–199. [Google Scholar]
- 3.Alström S. Induction of disease resistance in common bean susceptible to halo blight bacterial pathogen after seed bacterization with rhizosphere pseudomonads. J Genet App Microbiol. 1991;37:495–501. [Google Scholar]
- 4.Alexandre G, Jacoud C, Faure D, Bally R. Population dynamics of a motile and a nonmotile Azospirillum lipoferum strain during rice root colonization and motility variation in the rhizosphere. FEMS Microbiol Ecol. 1996;19:271–278. [Google Scholar]
- 5.Persello-Cartieaux F, David P, Sarrobert C, Thibaud MC, Achouack W, Robaglia C, Nussaume L. Utilization of mutants to analyze the interaction between Arabidopsis thaliana and its naturally root-associated Pseudomonas. Planta. 2001;212:190–198. doi: 10.1007/s004250000384. [DOI] [PubMed] [Google Scholar]
- 6.Ongena M, Daayf F, Jacques P, Thonart P, Benhamou N, Paulitz TC, Cornelis P, Koedam N, Belanger RR. Protection of cucumber against Phytium root rot by fluorescent pseudomonads: Predominant role of induced resistance over siderophores and antibiosis. Plant Pathol. 1999;48:66–76. [Google Scholar]
- 7.Pieterse CM, van Wees SCM, Hoffland E, van Pelt JA, van Loon LC. Systemic resistance in Arabidopsis induced by biocontrol bacteria is dependent of salicylic acid accumulation and pathogenesis-related gene expression. Plant Cell. 1996;8:1225–1237. doi: 10.1105/tpc.8.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuhegger R, Ihring A, Gantner S, Bahnweg G, Knappe C, Vogg G, Hutzler P, Schmidt M, van Breusegem F, Eberl L. Induction of systemic plant resistance by N-acylhomoserine lactone-producing rhizosphere bacteria. Plant Cell Environ. 2006;29:909–918. doi: 10.1111/j.1365-3040.2005.01471.x. [DOI] [PubMed] [Google Scholar]
- 9.Selvadurai EL, Brown AE, Hamilton JTG. Production of indole-3-acetic acid analogues by strains of Bacillus cereus in relation to their influence on seedling development. Soil Biol Biochem. 1991;23:401–403. [Google Scholar]
- 10.Lebuhn M, Heulin T, Hartmann A. Production of auxin and other indolic and phenolic compounds by Paenibacillus polymyxa strains isolated from different proximity to plant roots. FEMS Microbiol Ecol. 1997;22:325–334. [Google Scholar]
- 11.Arkhipova TN, Veselov SU, Melentiev AI, Mertynenko EV, Kudoyarova GR. Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil. 2005;272:201–209. [Google Scholar]
- 12.Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX, Paré PW, Kloepper JW. Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci USA. 2003;100:4927–4932. doi: 10.1073/pnas.0730845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.López-Bucio J, Campos-Cuevas JC, Hernández-Calderón E, Velásquez-Becerra C, Farías-Rodríguez R, Macías-Rodríguez LI, Valencia-Cantero E. Bacillus megaterium rhizobacteria promote growth and alter root system architecture through an auxin and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol Plant Microb Interact. 2007;20:207–217. doi: 10.1094/MPMI-20-2-0207. [DOI] [PubMed] [Google Scholar]
- 14.Aloni R, Aloni E, Laghans M, Ullrich CI. Role of cytokinin and auxin in shaping root architecture: Regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann Bot. 2006;97:883–893. doi: 10.1093/aob/mcl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salamone GIE, Hynes RK, Nelson LM. Cytokinin production by plant growth promoting rhizobacteria and selected mutants. Can J Microbiol. 2001;47:404–411. doi: 10.1139/w01-029. [DOI] [PubMed] [Google Scholar]
- 16.Nieto KF, Frankenberger WT. Microbial production of cytokinins. Soil Biochem. 1990;6:191–248. [Google Scholar]
- 17.Kakimoto T. Perception and signal transduction of cytokinins. Annu Rev Plant Biol. 2003;54:605–627. doi: 10.1146/annurev.arplant.54.031902.134802. [DOI] [PubMed] [Google Scholar]
- 18.Higuchi M, Pischke MS, Mähönen AP, Miyawaki K, Hashimoto Y, Seki M, Kobayashi M, Shinozaki K, Kato T, Tabata S, Helariutta Y, Sussman MR, Kakimoto T. In planta functions of the Arabidopsis cytokinin receptor family. Proc Natl Acad Sci USA. 2004;101:8821–8826. doi: 10.1073/pnas.0402887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishimura C, Ohashi Y, Sato S, Kato T, Tabata S, Ueguchi C. Histidine kinase homologs that act as cytokinin receptors posses overlapping functions in the regulation of shoot and root growth in Arabidopsis. Plant Cell. 2004;16:1365–1377. doi: 10.1105/tpc.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mähönen AP, Bishopp A, Higuchi M, Nieminen KM, Kinoshita K, Törmäkangas K, Ikeda Y, Oka A, Kakimoto T, Helariutta Y. Cytokinin signaling and its inhibitor AHP6 regulate cell fate during vascular development. Science. 2006;311:94–98. doi: 10.1126/science.1118875. [DOI] [PubMed] [Google Scholar]
- 21.Smalle J, Kurepa J, Yang P, Babychuk E, Kushnir S, Durski A, Vierstra RD. Cytokinin growth responses in Arabidopsis involve the 26S proteosome subunit RPN12. Plant Cell. 2002;14:17–32. doi: 10.1105/tpc.010381. [DOI] [PMC free article] [PubMed] [Google Scholar]