Abstract
We recently found that nuclei take different intracellular positions depending upon dark and light conditions in Arabidopsis thaliana leaf cells. Under dark conditions, nuclei in both epidermal and mesophyll cells are distributed baso-centrally within the cell (dark position). Under light conditions, in contrast, nuclei are distributed along the anticlinal walls (light position). Nuclear repositioning from the dark to light positions is induced specifically by blue light at >50 µmol m−2 s−1 in a reversible manner. Using analysis of mutant plants, it was demonstrated that the response is mediated by the blue-light photoreceptor phototropin2. Intriguingly, phototropin2 also seems to play an important role in the proper positioning of nuclei and chloroplasts under dark conditions. Light-dependent nuclear positioning is one of the organelle movements regulated by phototropin2. However, the mechanisms of organelle motility, physiological significance, and generality of the phenomenon are poorly understood. In this addendum, we discussed how and why nuclei move depending on light, together with future perspectives.
Key words: actin, Arabidopsis, blue light, cytoskeleton, nuclear positioning, nucleus, phototropin
Introduction
Nuclear positioning is a well-known phenomenon in plant cells and plays important roles in developmental events such as cell division and cell elongation.1,2 However, a new aspect of nuclear positioning was reported in 1993: nuclei change their intracellular positions in response to incident light in prothallial cells of the fern Adiantum capillus-veneris.3,4 This unique response was termed “light-dependent nuclear positioning”. Since the response was observed in cells that exhibit neither cell division nor cell elongation, it was suggested that light-dependent nuclear positioning plays some physiologically important role in cells exposed to light. However, the mechanisms of motility, physiological significance, and generality of the light-dependent nuclear positioning have not yet been elucidated in detail to date. We currently set out to dissect this fascinating phenomenon in higher plants and have recently found that nuclei in mature leaf cells of the spermatophyte Arabidopsis thaliana are differently distributed under dark and light conditions.5 In this addendum, we introduce basic features of light-dependent nuclear positioning in A. thaliana leaf cells and discuss the mechanisms of motility and physiological significance of the response, together with future perspectives.
Basic Features of Light-Dependent Nuclear Positioning in Arabidopsis thaliana
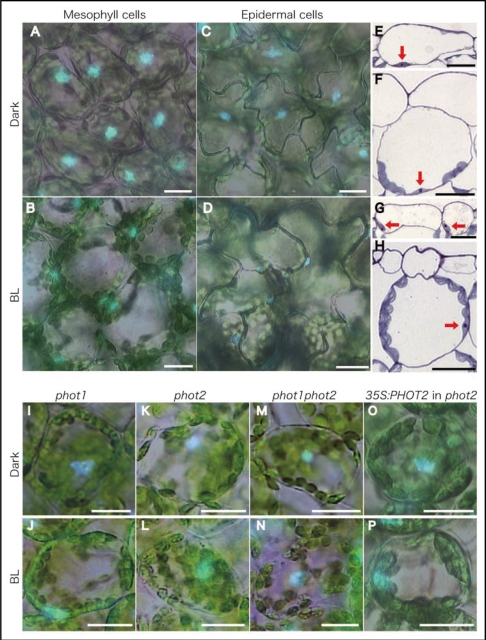
We initially observed the intracellular distribution of nuclei in mesophyll cells under dark and light conditions. Under dark conditions, nuclei were located baso-centrally within the cell, termed the dark position (Fig. 1A and F). Chloroplasts were distributed along the inner periclinal walls and the lower half of the anticlinal walls. Under 100 µmol m−2 s−1 of blue light, in contrast, nuclei were located along the anticlinal walls, termed the light position (Fig. 1B and H). Chloroplasts were distributed along the anticlinal walls, known as the chloroplast avoidance response.6 Nuclear repositioning was also observed in epidermal cells, which have no chloroplasts (Fig. 1C–E and G), suggesting that the nucleus can move without any involvement by chloroplasts. Nuclear repositioning from the dark to the light positions was specifically induced within a few hours of blue light irradiation at >50 µmol m−2 s−1, and was a reversible response. Furthermore, from the analysis of phototropin mutants and the complementation analysis of the phot2 mutant (Fig. 1I–P), we verified that the response was mediated by phototropin2 (phot2), which is known to mediate the chloroplast avoidance response.7,8 Additionally, we noticed that some nuclei and chloroplasts in the phot2 mutant were abnormally distributed along the anticlinal walls and outer periclinal walls, even under dark conditions. Similar results have been reported in Adiantum capillus-veneris.9 Thus, phot2 appears to play an important role in the proper positioning of nuclei and chloroplasts under both dark and light conditions in Arabidopsis and Adiantum.
Figure 1.
Distribution of nuclei in leaf cells of Arabidopsis thaliana in darkness and under blue light. After dark treatment for 16 h (Dark), leaves of wild-type plants (A–H), phototropin mutants phot1 (I and J), phot2 (K and L), phot1phot2 (M and N), and transgenic plant expressing 35S:PHOT2 gene in the phot2 mutant (O and P) were irradiated with blue light (470 nm, 100 µmol m−2 s−1) for 5 h (BL). Nuclei were stained with Hoechst33342, then bright-field and fluorescence images are demonstrated as merged pictures (A–D and I–P). Transverse sections of adaxial part of the leaves were stained with 0.5% toluidine blue (E–H). Each arrow indicates the position of the nucleus. Bars show 20 µm.
How do Nuclei Move in Response to Light?
We are investigating which cytoskeletal elements are involved in light-dependent nuclear positioning in A. thaliana. When actin filaments in epidermal cells kept under dark conditions were visualized by staining with Alexa488-phalloidin, thick, long actin bundles seemed to interact with the nucleus at the cell base. Conversely, under 100 µmol m−2 s−1 of blue light, actin bundles in the vicinity of the nucleus were apparently positioned along the anticlinal walls, together with the nucleus. LatranculinB, an actin-depolymerizing reagent, disrupted the actin bundles and caused the complete inhibition of light-dependent nuclear positioning in both epidermal cells and mesophyll cells. A possible interaction of actin filaments with nuclei has been already demonstrated in leaf cells of A. thaliana.10 These results suggest that reorganization of actin cytoskeleton plays an important role in light-dependent nuclear positioning; which also appears to be the case in the blue-light-induced chloroplast avoidance response.6 Thus, there are many similarities between light-dependent nuclear positioning and the chloroplast avoidance response with regard to the relevant photoreceptor (phot2), effective fluence rate (>50 µmol m−2 s−1 in the former, >32 µmol m−2 s−1 in the latter), and the responsible cytoskeletal elements.6–8,10,11 Plant cells may use very similar signaling pathways for the two different types of organelle movement. However, since it has been reported that chup1 (chloroplast unusual positioning1) mutant exhibits no chloroplast relocation movement but normal distribution of other organelles including nuclei, peroxisomes, and mitochondria,12 some components must be specific to each response. This suggestion may be supported by the fact that nuclei in epidermal cells, which lack chloroplasts, can respond to light (Fig. 1C–E and G). It would be worth testing whether the nuclear positioning is induced in the chup1 mutant.
Recently, Kadota and his colleagues have presented excellent results on investigations into the mechanisms of motility involved in chloroplast photorelocation movements.13,14 Upon the accumulation or avoidance response, chloroplasts migrate using short actin filaments (cp-actin), which newly appear at the leading edge of each chloroplast.13,14 The cp-actin is never observed in the chup1 mutant. In contrast, as described above, nuclei seem to migrate using heavily bundled actin filaments. Consequently, although the motility system for light-dependent nuclear positioning has not been fully elucidated, nuclei most likely move independently of chloroplasts using a nuclear specific mechanism implicating thick actin bundles.Further investigation into how myosin is involved in the actin reorganization and generation of driving force15,16 and whether Ca2+ regulates the reorganization17 through Ca2+-sensitive actin binding proteins such as villin,18 is required.
Why do Nuclei Move in Response to Light?
The physiological significance of light-dependent nuclear positioning also requires further investigation. We assume that it is one of the defensive responses for reducing DNA damage caused by excess light, and UV stress, from the two following points: (1) light-depenactin dent nuclear positioning is induced by so-called “strong light”, and (2) nuclei move to the anticlinal walls parallel to the direction of incident light minimizing the surface area directly facing the light. This assumption is supported by the fact that the chloroplast avoidance response genuinely functions in reducing photodamage caused by excess light,19 although current investigations in our lab seek to verify this possibility. Another possible function is that the response might be needed to polarize cell elongation in leaf palisade cells. In A. thaliana leaves grown under low-fluence-rate light, palisade cells become more spherical.20 Under high-fluence-rate light, in contrast, palisade cells become elongated in the adaxial/abaxial direction.20 Involvement of phototropins in the polar growth of palisade cells has been suggested by Kozuka et al.21 Therefore, relocation of nuclei from the cell bottom to the anticlinal walls might be responsible for the state shift from the nonpolar state to the polar state in palisade cells. Finally, light-dependent nuclear positioning might contribute to the efficient nuclear accumulation of photoreceptor molecules such as phytochromes. Phytochromes are known to translocate into the nucleus to interact with nuclear proteins.22 Nuclei move from the cell bottom to the anticlinal walls so that photoreceptors can efficiently translocate into the nuclei. Again, this requires further testing.
Perspectives
We have succeeded in partial characterization of blue-lightdependent nuclear positioning in A. thaliana leaf cells. However, we have not been able to follow the response in real time, due to the difficulty in observing transparent nuclei in living cells. Real-time observation of the response by visualizing nuclei, for example with GFP, is required for a better understanding of the response. This will enable us to precisely analyze the signaling pathway intervening between the light-perception by phot2 and the induction of nuclear movement, and it would become possible to isolate specific mutants defective in each step of the pathway. Moreover, rigorous comparison of the mechanisms of organelle motility for light-dependent nuclear positioning with those for the chloroplast avoidance response should elicit organelle-specific regulatory mechanisms of cytoskeleton. Now, we believe that light-dependent nuclear positioning is cell biologically and photobiologically a very attractive subject.
Acknowledgements
We thank Dr. Louis John Irving for critical reading of the manuscript.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/5213
References
- 1.Britz SJ. Chloroplast and nuclear migration. Encycl Plant Physiol. 1979;7:170–205. [Google Scholar]
- 2.Ketelaar T, Faivre-Moskalenko C, Esseling JJ, de Ruijter NCA, Grierson CS, Dogterom M, Emons AMC. Positioning of nuclei in Arabidopsis root hairs: An actin-regulated process of tip growth. Plant Cell. 2002;14:2941–2955. doi: 10.1105/tpc.005892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kagawa T, Wada M. Light-dependent nuclear positioning in prothallial cells of Adiantum capillus-veneris. Protoplasma. 1993;177:82–85. [Google Scholar]
- 4.Kagawa T, Wada M. Polarized light induces nuclear migration in prothallial cells of Adiantum capillus-veneris L. Planta. 1995;196:775–780. [Google Scholar]
- 5.Iwabuchi K, Sakai T, Takagi S. Blue light-dependent nuclear positioning in Arabidopsis thaliana leaf cells. Plant Cell Physiol. 2007;48:1291–1298. doi: 10.1093/pcp/pcm095. [DOI] [PubMed] [Google Scholar]
- 6.Wada M, Kagawa T, Sato Y. Chloroplast movement. Annu Rev Plant Biol. 2003;54:455–468. doi: 10.1146/annurev.arplant.54.031902.135023. [DOI] [PubMed] [Google Scholar]
- 7.Jarillo JA, Gabrys H, Capel J, Alonso JM, Ecker JR, Cashmore AR. Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature. 2001;410:952–954. doi: 10.1038/35073622. [DOI] [PubMed] [Google Scholar]
- 8.Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M. Arabidopsis NPL1: A phototropin homolog controlling the chloroplast high light avoidance response. Science. 2001;291:2138–2141. doi: 10.1126/science.291.5511.2138. [DOI] [PubMed] [Google Scholar]
- 9.Tsuboi H, Suetsugu N, Kawai-Toyooka H, Wada M. Phototropins and neochrome1 mediate nuclear movement in the fern Adiantum capillus-veneris. Plant Cell Physiol. 2007;48:892–896. doi: 10.1093/pcp/pcm057. [DOI] [PubMed] [Google Scholar]
- 10.Kandasamy MK, Meagher RB. Actin-organelle interaction: Association with chloroplast in Arabidopsis leaf mesophyll cells. Cell Motil Cytoskel. 1999;44:110–118. doi: 10.1002/(SICI)1097-0169(199910)44:2<110::AID-CM3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 11.Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K. Arabidopsis nph1 and npl1: Blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA. 2001;98:6969–6974. doi: 10.1073/pnas.101137598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oikawa K, Kasahara M, Kiyosue T, Kagawa T, Suetsugu N, Takahashi F, Kanegae T, Niwa Y, Kadota A, Wada M. Chloroplast unusual positioning1 is essential for proper chloroplast positioning. Plant Cell. 2003;15:2805–2815. doi: 10.1105/tpc.016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadota A, Yamada N, Sato Y, Oikawa K, Nakai M, Ogura Y, Kasahara M, Kagawa T, Suetsugu N, Wada M. Role of actin in the chloroplast photorelocation movement of Arabidopsis. Plant Cell Physiol Supp. 2006;47:1aH02. [Google Scholar]
- 14.Yamada N, Suetsugu N, Wada M, Kadota A. Role of short chloroplast actin filaments in the chloroplast photorelocation movement of Arabidopsis. Plant Cell Physiol Supp. 2007;48:1pC01. [Google Scholar]
- 15.Holweg C, Nick P. Arabidopsis myosin XI mutant is defective in organelle movement and polar auxin transport. Proc Natl Acad Sci USA. 2004;101:10488–10493. doi: 10.1073/pnas.0403155101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Paves H, Truve E. Myosin inhibitors block accumulation movement of chloroplasts in Arabidopsis thaliana leaf cells. Protoplasma. 2007;230:165–169. doi: 10.1007/s00709-006-0230-y. [DOI] [PubMed] [Google Scholar]
- 17.Harada A, Shimazaki K. Phototropins and blue light-dependent calcium signaling in higher plants. Photochem Photobiol. 2007;83:102–111. doi: 10.1562/2006-03-08-IR-837. [DOI] [PubMed] [Google Scholar]
- 18.Staiger CJ, Blanchoin L. Actin dynamics: Old friends with new stories. Curr Opin Plant Biol. 2006;9:554–562. doi: 10.1016/j.pbi.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Kasahara M, Kagawa T, Oikawa K, Suetsugu N, Miyao M, Wada M. Chloroplast avoidance movement reduces photodamage in plants. Nature. 2002;420:829–832. doi: 10.1038/nature01213. [DOI] [PubMed] [Google Scholar]
- 20.Tsukaya H. Leaf shape: Genetic controls and environmental factors. Int Dev Biol. 2005;49:547–555. doi: 10.1387/ijdb.041921ht. [DOI] [PubMed] [Google Scholar]
- 21.Kozuka T, Kong SG, Nagatani A. Regulation of polar cell elongation in palisade tissue by phototropins. Plant Cell Physiol Supp. 2007;48:2aJ02. [Google Scholar]
- 22.Nagatani A. Light-regulated nuclear localization of phytochromes. Curr Opin Plant Biol. 2004;7:708–711. doi: 10.1016/j.pbi.2004.09.010. [DOI] [PubMed] [Google Scholar]