Abstract
Although considerable researches have been conducted on the physiological responses to plant iron (Fe) deficiency stress in dicotyledonous plants, much still needs to be learned about the regulation of these processes. In the present research, red clover was used to investigate the role of root phenolics accumulation in regulating Fe-deficiency induced Fe(III) chelate reductase (FCR). The root FCR activity, IAA and phenolics accumulation, and also the phenolics secretion were greatly increased by the Fe deficiency treatment. The application of TIBA (2,3,5-triiodobenoic acid) to the stem, an IAA polar transport inhibitor, which could decrease IAA accumulation in root, significantly inhibited the FCR activity, but did not effect root phenolics accumulation and secretion, suggesting that IAA itself did not involve in root phenolics accumulation and secretion. In contrast, the Fe deficiency treatment significantly decreased the root IAA-oxidase activity. Interestingly the phenolics extracted from roots inhibited IAA-oxidase activity in vitro, and this inhibition was greater with phenolics extracted from roots of Fe deficient plants than that from Fe sufficient plants, indicating that the Fe deficiency-induced IAA-oxidase inhibition probably caused by the phenolics accumulation in Fe deficient roots. Based on these observations, we propose a model where under Fe deficiency stress in dicots, an increase in root phenolics concentrations plays a role in regulating root IAA levels through an inhibition of root IAA oxidase activity. This response, leads to, or at least partially leads to an increase in root IAA levels, which in turn help induce increased root FCR activity.
Key Words: Fe deficiency, ferric chelate reductase, phenolics, Trifolium pretense
Introduction
Fe deficiency is one of the most limiting factors affecting crop production in calcareous soils. However, many native plant species and genotypes termed Fe-efficient plants have evolved different strategies to avoid chlorosis caused by Fe deficiency. Romheld and Marschner had classified these strategies as Strategy I in nongraminaceous monocots and dicots, and Strategy II in graminaceous monocots. Because this study involves a dicot, we will not discuss Strategy II further.1
Strategy I plants respond to Fe-deficient stress by inducing several processes, including inducing a plasma membrane Fe(III) chelate reductase (FCR), proton extrusion and in most instances accompanied by the release of phenolic compounds.2,6 The induced phenolics secretion is probably related to changes in the phenolics content in plant tissues. It has long been known that various phenolic compounds can play a role in regulating IAA metabolism and IAA polar transport in plant.3,7,8 For example, a derivative of 7,4′-dihydroxyflavone (DHF) as well as free DHF were shown in in vitro assays to strongly inhibit enzyme-catalyzed IAA breakdown at concentrations found in root tissues, while formononetin, an isoflavonoid, can accelerate the enzyme-catalyzed breakdown of IAA.7 In addition, increased auxin transport was found in the Arabidopsis transparent testa4 (tt4) mutant which produces no flavonoids.9–12 Therefore, changes in the phenolics content in plant tissues probably are related to plant IAA levels changes.
It has been reported that Fe-deficient sunflower roots contained higher levels of IAA than the Fe-sufficient ones.1 Therefore, IAA has been implicated to play a role in triggering Fe-deficiency responses. Application of TIBA (2,3,5-triiodobenzoic acid) or CFM (2-chloro-9-hydroxyfluorenecarboxylic acid-9-methylester), two inhibitors of polar IAA transport, to Fe-deficient plants markedly inhibited FCR activity,13 proton extrusion and subapical root hairs development.14 Meanwhile, Fe-sufficient plants treated with IAA or IBA (indol-3-butylric acid, another natural auxin), have been demonstrated to closely mimic the root FCR activity and root morphology of Fe-deficient plants.15,16 The mechanism by which IAA acts is related to changes in IAA concentration at the specific site of action, which is controlled by its biosynthesis, metabolism and transport.17 Although the root can also synthesize the IAA, the shoot derived IAA is still an important source in determining root IAA levels. Therefore, the IAA basipetal transport and metabolism are two important elements determining root IAA concentrations. Therefore, if the phenolics content in Fe deficient root was changed, it was probably involved in regulating the Fe deficiency induced responses through the link in root phenolics content changes and IAA-oxidase activity in response to Fe deficiency. The objective of the present research was focused on investigating this hypothetical link in the red clover (Trifolium pratense cv. Kenland).
Materials and Methods
Plant culture.
Red clover seeds were soaked in distilled-deionized water overnight. After rinsed three times, the seeds were transferred to an incubator at 25°C for germination in dark. The germinated seeds were transferred to a plastic mesh tray placed over a container filled with 0.5 mM CaCl2 solution. The solution was renewed daily. Three days later, the solution was replaced with one fifth strength of complete nutrient solution. After 12 days, uniform seedlings were transplanted to 1 L pots (four holes per seedling holder, and three seedlings per hole) filled with aerated, full strength complete nutrient solution. The nutrient solution had the following macro-nutrient composition: 3 mM Ca(NO3)2, 0.5 mM MgSO4, 0.3 mM NaH2PO4 and 0.5 mM K2SO4. Micronutrient concentrations were 3 µM H3BO3, 0.4 µM ZnSO4, 0.2 µM CuSO4, 0.5 µM MnCl2, 0.01 µM (NH4)6(MO7)24 and 20 µM Fe(III)-EDTA. The solution pH was adjusted to 6.5 using 1 M NaOH. Nutrient solutions were renewed every other day. After certain days of cultivation as indicated in the different experiments, plants were subjected to different treatments. All treatments had three replicates and all experiments were repeated independently at least two times, and the results from one set of representative experiments were presented. Plants were grown in a greenhouse with an average temperature of 28/23°C, relative humidity of 50/80%, and photo-cycle of 14 h/10 h (day/night), and average daytime photosynthetically active radiation between 500 and 1000 µmol photons m−2s−1.
TIBA (2,3,5-triiodobenzoic acid) and NPA (N-1-naphthylphthalamic acid) treatments.
The 23-day-old plants were subjected to the following treatments: (1) Control, the plants still cultured in complete nutrient solution; (2) −Fe, the plants transferred to the same nutrient solution lacking Fe; (3) −Fe + TIBA, 2 mM TIBA dissolved in lanoline was applied to the stem of the −Fe grown plant below the cotyledons, and was reapplied every three days. NPA was also applied and treated in the same way as TIBA.
Exogenous IAA treatment.
The 23-day-old plants were subjected to the following treatments: (1) Control; (2) −Fe; (3) Control + IAA, the plants were transferred to complete nutrient solution contained 2 × 10−7 M IAA; (4)−Fe + IAA, the plants were transferred to −Fe nutrient solution containing 2 × 10−7 M IAA. All the treatment solutions were renewed daily.
The measurement phenolic compounds secretion.
The concentration of total phenolic compounds in the nutrient solutions were determined colorimetrically at 750 nm using Folin-Ciocalteu's reagent according to Singleton and Rossi.18 The concentration of the total phenolic compounds was expressed in terms of molar equivalent of gallic acid.
Measurement of phenolics content in roots.
The phenolics content in roots was measured according to Siddhuraju and Becker4 with some brief modifications. Briefly, one gram of root was ground in a mortar to a fine powder with liquid N2 and was extracted with 8 mL of 80% methanol in a sonication bath for 25 min. The solution was centrifuged at 5000 g for 10 min. The total phenolics concentration in the supernatant was determined colorimetrically at 750 nm using Folin-Ciocalteu's reagent.18
Analysis of the difference of the phenolics compositions between Fe-deficient and Fe-sufficient roots.
The phenolics was extracted from 0.5 gram of Fe deficient or Fe sufficient root as described in former Section. The methanol extract was passed through a 0.45-µm membrane filter, and 10 µL was used for HPLC analysis (Beckman). The samples were injected into a Beckman C18 (ODS) 5 µm, 4.6 × 250 mm HPLC column heated to 30°C. The mobile phase consisted of (A) three parts 0.02M NH4H2PO4 to one part 100% methanol (V:V) and (B) 100% methanol. The flow rate was 0.8 mL/min, with a gradient profile consisting of A with the following proportions (v/v) of B: 0–15 min, 5%; 15–20 min, 5–35% B; 20–35 min, 35% B; 35–45 min, 35–100% B; 45–50 min, 100% B; 50–52 min, 100–5% B. The phenolic compounds in root extracts were identified by UV spectra at 280 nm.
Measurement of IAA-oxidase activity.
One gram of fresh root sample was homogenized in 5 ml of ice-cold 100 mM phosphate buffer (NaH2PO4/Na2HPO4, pH 7.0). The homogenate was centrifuged at 15000 g for 20 min at 4°C and the supernatant which contains the enzyme extract, was used for IAA oxidase activity analysis according to the methods of Beffa et al.19 Briefly, reaction mixtures contained 3 ml of 100 mM phosphate buffer (NaH2PO4/Na2HPO4, pH 7.0), 2 ml of 1 mM MnCl2, 2 ml of 1 mM 2, 4-dichlorophenol (DCP), 2 ml of 1 mM IAA and 1 ml of enzyme extract, and were kept at 25 ± 0.5°C. After reaction for 40 min, 3 mL of the reaction mixture was mixed with 2 mL of Salkowski reagent. The Salkowski reagent was prepared according to the method of Sarwar et al.20 The mixture was allowed to stand for 30 min for color development, and the absorbance was recorded at 530 nm. The enzyme activity was expressed as µg of oxidized IAA per g fresh root per minute.
Effect of extracted root phenolics on IAA-oxidase activity.
After 13 days of growth on −Fe media, roots from both Fe-deficient and Fe-sufficient plants were harvested, and phenolics extracted as described in former Section. The extractant was dried at 40°C in a rotary evaporator. Residues were dissolved in 100 mM phosphate buffer (NaH2PO4/Na2HPO4, pH 7.0) and stored at 4°C for later use. The quantity of the total phenolics in phosphate buffer was measured colorimetrically using Folin-Ciocalteu's reagent.
Crude IAA oxidase enzyme extract was prepared from 10 g of roots from 38-day-old Fe-sufficient plants as described in former Section. Different concentrations of phenolics isolated from roots of Fe-sufficient and Fe-deficient plants were added to the reaction mixtures. The rate of phenolics-induced inhibition of IAA-oxidase activity was calculated according to the following equation:
Inhibited rate (%) = (Ab−Ap)/Ab × 100
where Ab is the IAA-oxidase activity with no root-extracted phenolics, and Ap is that with root-extracted phenolics.
The measurement of IAA content in root.
IAA extraction, purification and assay were modified from Yang et al.21 Briefly, about 0.500 g roots was homogenized in 3 ml prechilled 80% methanol on ice in weak light condition, with about 0.1 g polyvinylpyrrolidone (PVP) added. Homogenate was centrifuged at 5000 rpm for 10 min at 4°C, debris was cleaned with 0.5 ml prechilled 80% methanol and centrifuged. Supernatant was combined and purified with C18 columns (C18Sep-Park Cartridge, Waters Corp., Millford, MA). Then 300 µL of the extract was dried under N2 gas, and redissolved in 200 µl methanol. The IAA in the methanol solution was methylized with diazomethane, followed by drying under N2 gas and redissolved in 300 µl phosphate buffer solution (PBS, 1.3 mM NaH2PO4, 8.7 mM Na2HPO4, 0.14 M NaCl, pH 7.4) for enzyme-linked immunosorbent assay (ELISA). The ELISA kits were purchased from Phytohormone Research Institute, Nanjing Agricultural University, China. Absorbance of the developed color was measured at 490 nm using a microplate reader. The recovery rate of this immunoassay for IAA is 76%.
Determination of FCR activity.
FCR activity was determined according to Grusak.22 Briefly, one gram of the excised roots (less than 10 cm from the root tips) was placed in an Erlenmeyer flask filled with 50 ml of an assay solution consisting of 0.5 mM CaSO4, 0.1 mM Mes (4-morpho-lineethanesulfonic acid), 0.1 mM BPDS (4,7-diphenyl-1,10 -phenanhroline-disufonic acid) and 100 µM Fe EDTA at pH 5.5 adjusted by 1 M NaOH. The flasks were placed in a dark room at 25°C for 1 h, with periodically hand-swirling at 15 min intervals. The absorbance of the assay solutions was recorded by a spectrophotometer at 535 nm, and the concentration of Fe(II)[BPDS]3 was quantified using an extinction coefficient of 22.14 mM−1 cm−1.
Results
Effect of Fe deficiency on root phenolics secretion and accumulation.
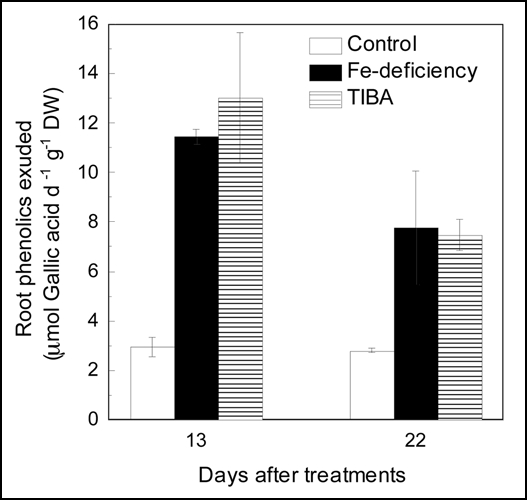
Most Fe-efficient dicot plants secret phenolic compounds under Fe-deficient conditions,1,23,24 and we show here that this also occurs in red clover (Fig. 1). Compared with that of Fe sufficient roots, the phenolics secretion of the Fe deficient roots was greatly increased (Fig. 1). Phenolics accumulation in root was also significantly increased by the Fe deficient treatment, showing about a 40% increase (Fig. 2A). When the stem was treated with TIBA, both phenolics secretion and accumulation in the roots of Fe deficient plant were not affected. The NPA treatment also did not affect these two Fe deficiency-induced responses (data not shown). In addition, The exogenous IAA treatment did not affect the phenolics accumulation both in Fe sufficient and Fe deficient roots too (Fig. 2B). These results suggest that root phenolics secretion and accumulation in response to Fe-deficiency was independent of IAA.
Figure 1.
Effects of Fe-deficiency and stem TIBA application on root phenolics secretion. The 23-day-old plants were subjected to following treatments: Control, plants cultured in complete nutrient solution; −Fe, plants cultured in nutrient solution lacking Fe; −Fe + TIBA, TIBA was applied to the stem of the −Fe grown plants Root phenolics secretion is expressed in terms of molar equivalents of gallic acid. Error bars show standard deviation (n = 3).
Figure 2.
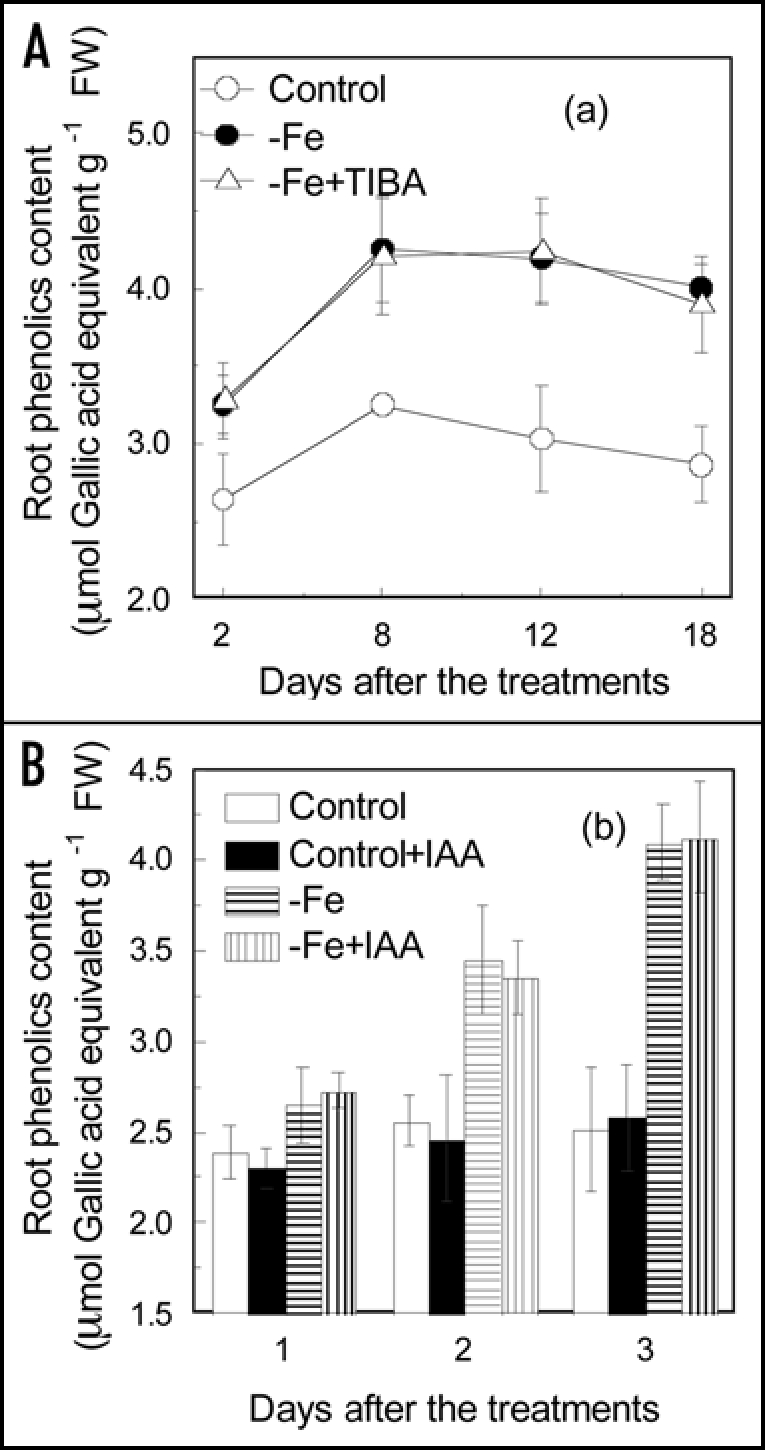
Effect of TIBA (A) and exogenous IAA (B) treatments on phenolics accumulation in roots. Twenty-three-day-old plants were used in this investigation. Control, Plants cultured in complete nutrient solution; −Fe, Plants cultured in nutrient solution lacking Fe; −Fe + TIBA, TIBA was applied to the stem of Fe deficient plants Control + IAA, Plants cultured in complete nutrient solution containing 0.2 µM IAA; −Fe + IAA, plants cultured in −Fe nutrient solution containing 0.2 µM IAA. The root content of total phenolic compounds is expressed in terms of molar equivalents of gallic acid. Error bars show standard deviation (n = 3).
Effects of Fe-deficiency and root extracted phenolics on root IAA-oxidase activity.
IAA-oxidase activity in the roots was strongly inhibited by the Fe deficiency, particularly as the plants became more Fe deficient (Fig. 3). An experiment investigating the effects of phenolics extracted from roots of Fe deficient plants on in vitro IAA-oxidase activity showed that phenolics strongly inhibited IAA oxidase activity (Fig. 4A). Interestingly, total phenolics extracted from roots of Fe deficient plants inhibited IAA oxidase activity stronger than that from roots of Fe sufficient plants at any particular concentration of phenolics (Fig. 4A). In addition, the inhibition of IAA-oxidase activity clearly increased as phenolics concentrations were increased in the reaction mixture from 20 to 400 µM, which corresponded to root phenolics concentrations of 1.2 to 24 mM (Fig. 4B). At a concentration of 70 µM, which approximately corresponded to the measured phenolics content of roots from Fe-deficient plants (about 4 mM) (Fig. 2), the inhibition of IAA oxidase activity was approx 60%.
Figure 3.
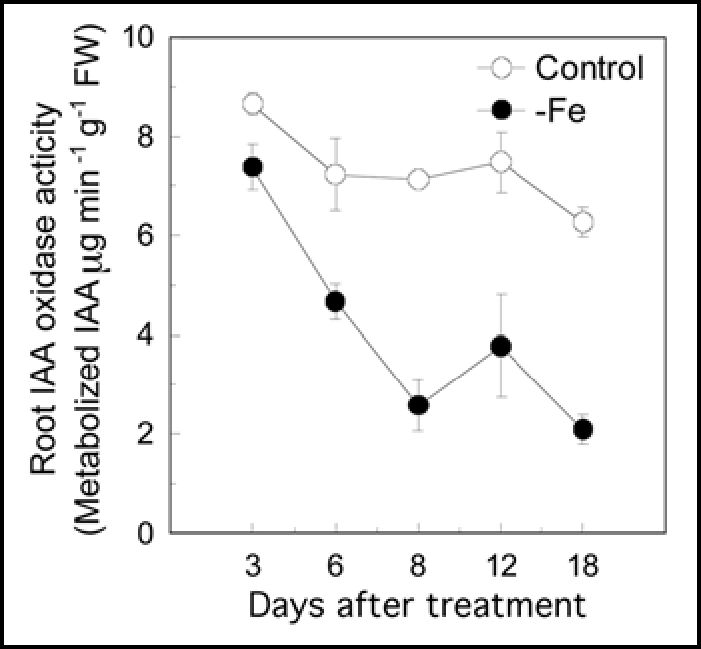
Effect of Fe deficiency on IAA-oxidase activity in the root. Twenty-three-day-old plants were used in this investigation. Control, Plants cultured in complete nutrient solution; −Fe, Plants cultured in nutrient solution lacking Fe. Error bars show standard deviation (n = 4).
Figure 4.
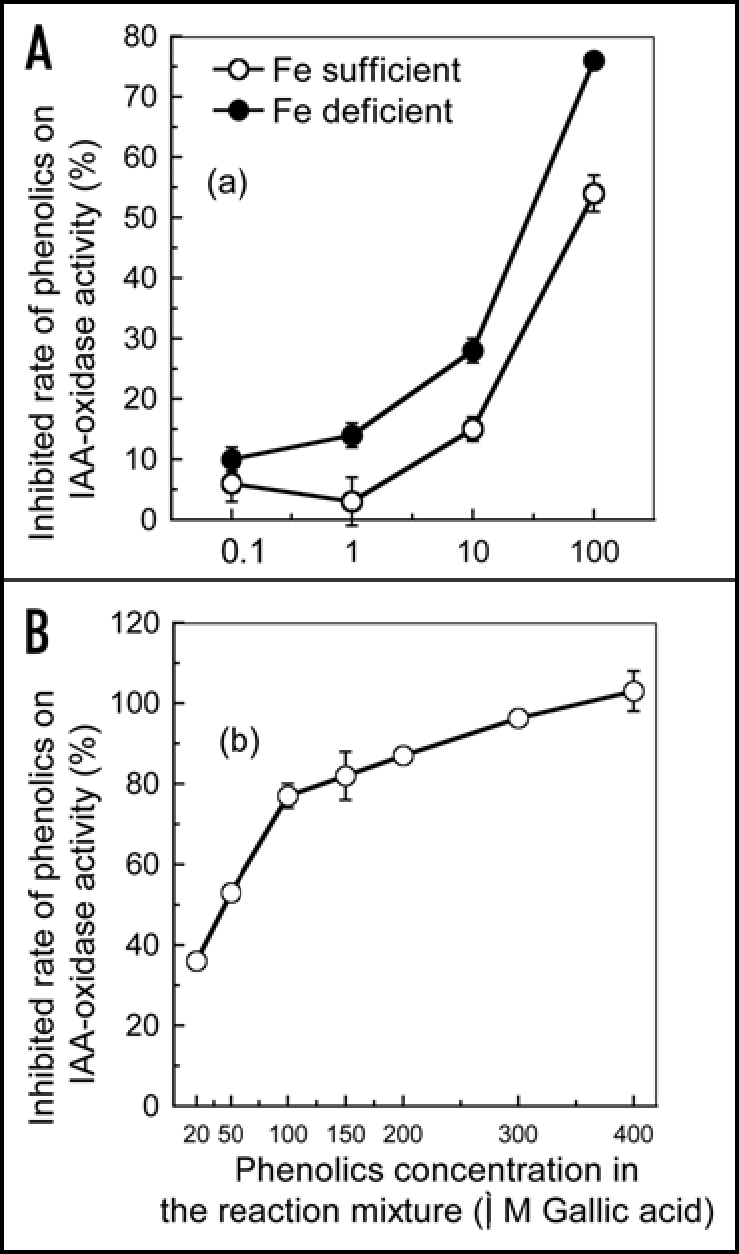
Effect of the phenolics extracted from the roots on root IAA oxidase activity. (A) Concentration dependence of the inhibition of root IAA oxidase activity by phenolic compounds isolated from roots of Fe deficient and Fe sufficient plants. Total phenolic concentration in the assay ranged from 0.1 to 100 µM; (B) Inhibition rates of root IAA oxidase activity by phenolic compounds isolated from roots of Fe deficient plants. Total phenolic concentration in the assay ranged from e 20 to 400 µM. Error bars show standard deviation (n = 3).
Effects of Fe-deficiency on the endogenous root IAA level.
On day three of Fe-deficient treatment, the root IAA content showed no difference between Fe deficient and sufficient plants. However, from the day six on, the root IAA contents in Fe deficient plants were remarkably higher than that of Fe sufficient plant (Fig. 5).
Figure 5.
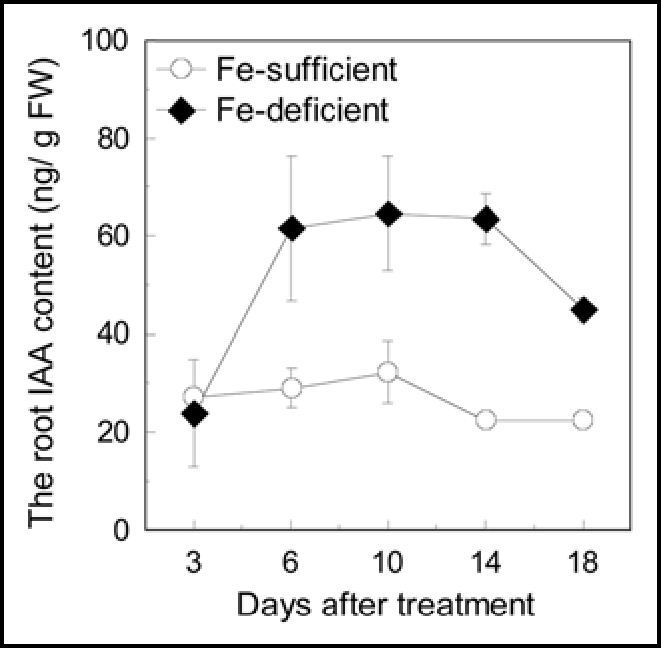
Effect of Fe deficiency on IAA content in the root. Twenty-three day-old plants were used in this investigation. Control, plants cultured in complete nutrient solution; −Fe, plants cultured in nutrient solution lacking Fe. Error bars show standard deviation (n = 4).
Effect of TIBA application on the patterns of Fe-deficiency induced FCR.
Root FCR activity was strongly induced by the imposition of Fe deficiency. When the stem was treated with TIBA (Fig. 6) or NPA (data not shown), root FCR activity progressively decreased, but it retained at a higher level than that in roots of control (+Fe grown) plants for the entire treatment period, suggesting that the IAA should be involved in regulating Fe deficiency-induced FCR activity.
Figure 6.
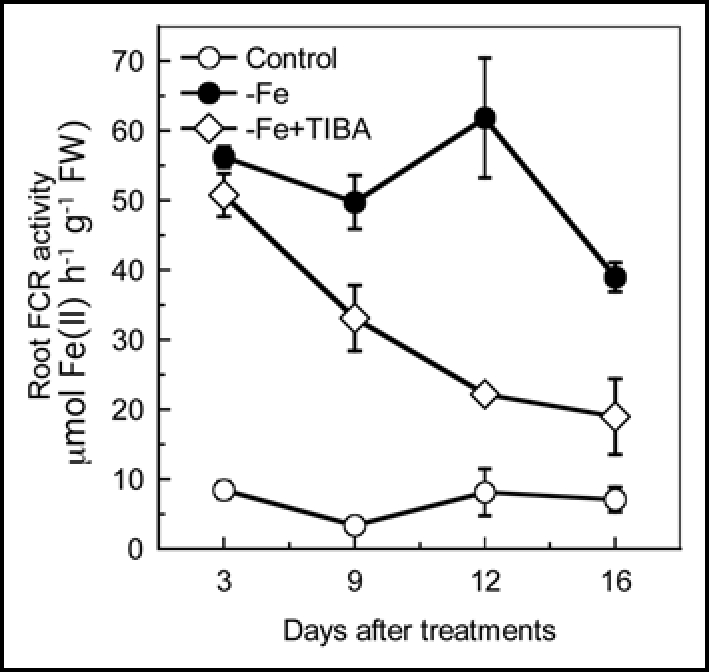
Effects of stem TIBA application on Fe deficiency induced FCR activity. The 23-day-old plants were subjected to following treatments: Control, plants cultured in complete nutrient solution; −Fe, plants transferred to −Fe nutrient solution; −Fe + TIBA, TIBA was applied to the stem of the −Fe plants. Error bars show standard deviation (n = 3).
Discussion
The accumulated phenolics in roots and their relationship with IAA-oxidase activity.
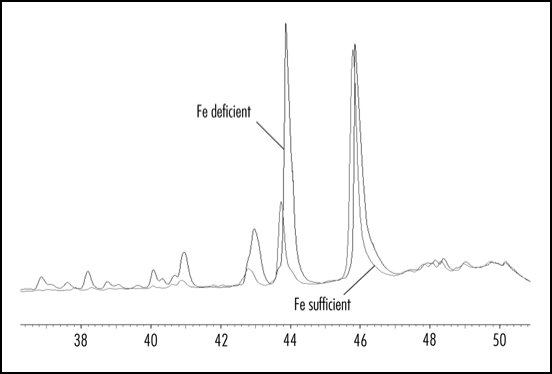
In plants, various phenolic compounds have been demonstrated to have the ability to regulate IAA-oxidase activity.3,7,8 and the IAA-oxidase activity should play an important role in regulating root IAA levels. As shown in Figure 3, root IAA-oxidase activity was significantly inhibited by the imposition of Fe deficiency in the plant. Thus we investigated whether this inhibition of root IAA oxidase activity was due to Fe deficiency-induced accumulation of phenolic in the root. This was done by investigating the ability of phenolics extracted from the root to affect enzyme-catalysed IAA breakdown in vitro using IAA-oxidase isolated from roots of Fe sufficient plants. As shown in Figure 4A, the phenolics extracted from roots of Fe-deficient and sufficient plants both inhibited IAA-oxidase activity in vitro, with phenolics extracted from roots of Fe deficient plants have a greater inhibitory effect than those from roots of Fe sufficient plants at the same concentration. The HPLC profiles of the extracted phenolics from Fe-sufficient and deficient roots showed that Fe deficiency definitely induced some new phenolic compounds and increased the content of some phenolics (Fig. 7). Therefore, it is highly possible that phenolics from Fe deficient roots might contain more active phenolic components that have higher inhibitory effect. In addition, the inhibitory effect of phenolics on IAA-oxidase activity increased as the phenolics concentration was increased (Fig. 4A and B). All of these results suggest that the inhibition of root IAA-oxidase activity in response to Fe deficiency may be due to both the increases in root phenolics levels and changes in phenolics compositions, and this relationship is likely linked to the IAA levels increases in the Fe-deficient root (Fig. 5). Of course, which compounds in the Fe-deficient root extracts are more effective in inhibiting auxin oxidase need further investigation by identifying the compounds and doing the bioassay of the individual compound purified.
Figure 7.
The HPLC profile difference of the phenolics composition between Fe-deficient and Fe-sufficient root. The phenolics was extract from the roots with 80% methanol, and then the extracts was analyzed by HPLC. Column, 5-µm Beckman C18 (ODS); flow, 0.8 ml min−1; detection, 280 nm.
It is worthy to mention here, that the phenolics content of Fe-deficient root had been increased at the second day of Fe-deficient treatment (Fig. 2), while the IAA content of Fe-deficient was still unaltered at the third day of Fe-deficient treatment (Fig. 5), suggesting that the occurrence of phenolics content change is an earlier event than IAA content change. This phenomenon interestingly fit the hypothesis that the phenolics accumulation in Fe-deficient root is, at least, one of the main factors resulting in the IAA level increases.
In contrast to the root, the phenolics content in the Fe-deficient shoot was lower than in the Fe-sufficient shoot (data not shown). Many phenolic compounds have been demonstrated to negatively regulate IAA transport.9,12 Therefore, the decreased phenolics content in shoot may also contribute to the IAA accumulation in the root through accelerating IAA transport from shoot. This hypothetical model will be investigated in future research.
Involvement of IAA in regulating induction of the root FCR by Fe deficiency.
Fe-deficient red clover roots had much higher IAA contents than Fe-sufficient roots (Fig. 5). The shoot derived IAA has been demonstrated a critically important signal molecule in regulating various root physiological responses.17,25 Application of TIBA (Fig. 6) or NPA clearly inhibited Fe-deficiency-inducible root FCR activity. Furthermore, decapitation of the shoot apex and any new expanded leaves, which will inhibit subsequent IAA synthesis in the shoot, also significantly inhibited root FCR activity in the Fe-deficient red clover, implying that the IAA is involved in regulating the increased expression of FCR capacity when the plant suffers from Fe deficiency. Therefore, it is highly possible that the Fe deficiency induced phenolic compounds secretion and FCR are differentially regulated.
Recently, Zheng et al. demonstrated that the root FCR in red clover was induced within one day, and displayed two distinct peaks of increased activity during 24 days of Fe-deficiency treatment.26 In addition, shoot apical decapitation inhibited the second cycle of increased FCR activity, but did not influence the first peak (on day three). In present research, we also found that the FCR activity was almost not inhibited by TIBA on day three (Fig. 6), and the IAA content in the same day was also show no difference between Fe-deficient and Fe-sufficient root (Fig. 5). In addition, the Fe-deficiency induced FCR activity following stem TIBA application was still significantly higher than the same activity in Fe sufficient plants, and so did the shoot apex decapitation.26 In addition, we also found that the exogenous IAA treatment to Fe-deficient root could significantly increase the FCR activity, but the same treatment to Fe-sufficient root can not mimic the FCR activity induced by Fe deficiency.27 Therefore, we hypothesize that IAA may be a secondary signal that, for example, may amplify the degree of later peak of root FCR gene expression or enhance translation of the transcribed mRNA, but is not the primary factor determining whether this FCR gene(s) is expressed or not. Of course, we still can not rule out that the root itself directly perceives the −Fe deficiency signal which results in the induction of the FCR as proposed in our earlier report.26 Besides, the enhancing effect of IAA on FCR activity may be also achieved by altering the root architecture, such as increasing the root hairs and lateral roots, both of which can increasing the root surface for ion uptake.
When roots were treated with exogenous IAA, the FCR activity in −Fe plant roots was significantly increased, but only a slight increase was seen in roots of Fe-sufficient plants with the same treatment.27 This result further supports the above hypothesis since if the IAA were the only signal determining Fe-deficiency induced FCR gene(s) expression, and then the root FCR activity should also be strongly stimulated by exogenous IAA in Fe sufficient plants. Nevertheless, the internal IAA and exogenous IAA pools could be functionally different in terms of regulating the physiological responses, which needs to be studied further.
The potential joint effect of internal phenolics levels and IAA in regulating iron-deficiency induced FCR.
The present research using stem TIBA application demonstrated that the IAA is involved in regulating Fe-deficiency-induced root FCR activity, but is not involved in root phenolics secretion and accumulation, suggesting that phenolics are differentially regulated from other Fe-deficiency responses. Application of NPA to the root-shoot junction can inhibit IAA transporter from shoot to root,5 in present research, we found that NPA had the same effect on Fe deficient responses (data not shown). This further supports the involvement of the IAA in regulating Fe-deficiency-induced responses. The combined observations that Fe deficiency inhibited root IAA oxidase activity (Fig. 3), and increased root phenolics content (Fig. 2) and endogenous root IAA content (Fig. 5), along with the observed inhibition of IAA oxidase activity by increasing concentrations of phenolics (Fig. 4), allow us to suggest the following model: When the plant suffers from Fe deficiency stress, the increase in root phenolics concentrations play a role in enhancing root IAA levels through at least partially through an inhibition of root IAA oxidase activity. This response contributes to increasing root IAA levels, which in turn help induce increased later peak of root FCR activity. These findings are helping to advance our understanding of the signaling pathways associated with the response of Strategy I plants to Fe deficiency.
Acknowledgements
This work was financially supported by Natural Science Foundation of China (Contract No.30625026) and the Program for New Century Excellent Talents in University (NCET-04-0554). Thanks are given to Dr. Leon. Kochian of US Plant, Soil and Nutrition Laboratory, USDA-ARS for his critical reading of the manuscript.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4502
References
- 1.Rmheld V, Marschner H. Mobilization of iron in the Rhizosphere of different plant species. Adv Plant Nutr. 1986;2:155–204. [Google Scholar]
- 2.Curie C, Briat JF. Iron transport and signaling in plants. Annu Rev Plant Biol. 2003;54:183–206. doi: 10.1146/annurev.arplant.54.031902.135018. [DOI] [PubMed] [Google Scholar]
- 3.Gortner WA, Kent MJ, Sutherland GK. Ferulic and p-coumaric acids in pineapple tissue as modifiers of pineapple indoleacetic acid oxidase. Nature. 1958;181:630. [Google Scholar]
- 4.Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J Agr Food Chem. 2003;51:2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- 5.Reed RC, Brady SR, Muday GK. Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol. 1998;118:1369–1378. doi: 10.1104/pp.118.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hell R, Stephan UW. Iron uptake, trafficking and homeostasis in plants. Planta. 2003;216:541–551. doi: 10.1007/s00425-002-0920-4. [DOI] [PubMed] [Google Scholar]
- 7.Mathesius U. Flavonoids induced in cells undergoing nodule organogenesis in white clover are regulators of auxin breakdown by peroxidase. J Exp Bot. 2001;52:419–426. doi: 10.1093/jexbot/52.suppl_1.419. [DOI] [PubMed] [Google Scholar]
- 8.Pilet PE. Effect of p-hydroxybenzoic acid on growth, auxin content and auxin catabolism. Phytochemistry. 1966;5:77–82. [Google Scholar]
- 9.Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, Taiz L, Muday GK. Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol. 2001;126:524–535. doi: 10.1104/pp.126.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buer CS, Muday GK. The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell. 2004;16:1191–1205. doi: 10.1105/tpc.020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy A, Peer WA, Taiz L. Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta. 2000;211:315–324. doi: 10.1007/s004250000300. [DOI] [PubMed] [Google Scholar]
- 12.Peer WA, Bandyopadhyay A, Blakeslee JJ, Makam SN, Chen RJ, Masson PH, Murphy AS. Variation in expression and protein localization of the pin family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell. 2004;16:1898–1911. doi: 10.1105/tpc.021501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C, Zhu X, Zhang F. Role of shoot in regulation of iron deficiency responses in cucumber and bean plants. J Plant Nutr. 2000;23:1809–1818. [Google Scholar]
- 14.De la Guardia MD, Alcántara E, Fernández M. Iron reduction by sunflower roots under iron stress. In: Crane FL, Morré DJ, Low H, editors. Proceedings NATO advanced research workshop on plasma membrane oxidoreductases in control of animal and plant growth. New York, NY: Plenum Press; 1988. p. 430. [Google Scholar]
- 15.Li X, Li C. Is ethylene involved in regulation of root ferric reductase activity of dicotyledonous species under iron deficiency. Plant Soil. 2004;261:147–153. [Google Scholar]
- 16.Landsberg EC. Hormonal regulation of iron-stress response in sunflower roots: A morphological and cytological investigation. Protoplasma. 1996;194:69–80. [Google Scholar]
- 17.Woodward AW, Bartel B. Auxin: Regulation, action, and interaction. Ann Bot. 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singleton VL, Rossi JAJ. Colorimetry of total phenolics with phosphomolybdic -phosphotungstic acid reagents. Am J Enol Viticult. 1965;16:144–158. [Google Scholar]
- 19.Beffa R, Martin HV, Pilet PE. In vitro oxidation of indoleacetic acid by soluble auxin-oxidases and peroxidases from maize roots. Plant Physiol. 1990;94:485–491. doi: 10.1104/pp.94.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarwar M, Arshad M, Martens DA, Frankenberger JWT. Trytophan-dependent biosynthesis of auxins in soil. Plant Soil. 1992;147:207–215. [Google Scholar]
- 21.Yang J, Zhang J, Wang Z, Zhu Q, Wang W. Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiol. 2001;127:315–323. doi: 10.1104/pp.127.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grusak MA. Whole-root iron(β)-reductase activity throughout the life cycle of iron-grown Pisum sativum L. (Fabaceae): Relevance to the iron nutrition of developing seeds. Planta. 1995;197:111–117. [Google Scholar]
- 23.Susin S, Abian J, Sanchez-beyes JA, Peleato ML, Abadia A, Gelpi E, Abadia J. Riboflavin 3′- and 5′-sulphate, two novel flavins accumulating in the roots of iron-deficient sugar beet (Beta vulgaris) J Biol Chem. 1996;268:20958–20965. [PubMed] [Google Scholar]
- 24.Wei LC, Loeppert RH, Ocumpaugh WR. Fe-deficiency stress response in Fe-deficiency resistant and susceptible subterranean clover: Important of induced H+ release. J Exp Bot. 1997;48:239–246. [Google Scholar]
- 25.Phellips IDJ. Apical dominance. Annu Rev Plant Physiol. 1975;26:341–367. [Google Scholar]
- 26.Zheng SJ, Tang CX, Arakawa Y, Masaoka Y. The responses of red clover (Trifolium pratense L.) to iron deficiency: A root Fe(III) chelate reductase. Plant Sci. 2003;164:679–687. [Google Scholar]
- 27.Jin CW, He YF, Tang CX, Wu P, Zheng SJ. Mechanisms of microbially enhanced Fe acquisition in red clover (Trifolium pretense L.) Plant Cell Environ. 2006;29:888–897. doi: 10.1111/j.1365-3040.2005.01468.x. [DOI] [PubMed] [Google Scholar]