Abstract
Previously, we reported that in Citrus plants, nitrate influx through the plasmalemma of roots cells follows a biphasic pattern, suggesting the existence of at least two different uptake systems, a high and low affinity transport system (HATS and LATS, respectively). Here, we describe a novel inducible high affinity transport system (iHATS). This new nitrate transport system has a high capacity to uptake nitrate in two different Citrus rootstocks (Cleopatra mandarin and Troyer citrange). The iHATS was saturable, showing higher affinity than constitutive high affinity transport system (cHATS) to the substrate NO3−. The Vmax for this saturable component iHATS was higher than cHATS, reaching similar values in both rootstocks.
Additionally, we studied the regulation of root NO3− uptake mediated by both HATS (iHATS and cHATS) and LATS. In both rootstocks, cHATS is constitutive and independent of N-status. Concerning the regulation of iHATS, this system is upregulated by NO3− and down-regulated by the N status and by NO3− itself when plants are exposed to it for a longer period of time. LATS in Cleopatra mandarin and Troyer citrange rootstocks is repressed by the N-status.
The use of various metabolic uncouplers or inhibitors indicated that NO3− net uptake mediated by iHATS and LATS was an active transport system in both rootstocks.
Key Words: Citrus, inducible high affinity transport system (iHATS), constitutive high affinity transport system (cHATS), nitrate uptake, regulation
Introduction
Plants can extract and use a wide range of inorganic and organic forms of nitrogen (N) from soils. However, except in agricultural systems fertilized with urea, nitrate (NO3−) and ammonium (NH4+) are believed to provide the bulk of the N resource available to the plants. Productivity in agricultural systems is highly dependent on the availability of N for uptake by roots. Roots of higher plants can absorb organic sources of nitrogen like amino acids and low molecular weight compounds,1–2 however mineral N is mainly acquired from the soil like ammonium and nitrate salts.
Physiological studies have demonstrated that powerful regulatory mechanism operate at the whole plant level, so that in the long term, nitrate uptake depends on internal factors related to N demand of the plant, rather than on nitrate availability in the soil volume. It has been shown for a number of plant species that influx of NO3− involves al least three different transport systems.3–4 When NO3− is available at low concentrations (<1 mM) two different system transports are operating, one is constitutive (cHATS) and the other is inducible (iHATS) and operates only after prior exposure to nitrate. Both systems follow saturable kinetic patterns and display low Km values. cHATS has a higher affinity for NO3−, but iHATS as an enhanced uptake capacity.5–7 An additional transporter, a LATS, exhibits linear non-saturable kinetics depending on increasing external nitrate concentration.5 In Arabidopsis and Brassica napus an inducible component for a nitrate LATS has been reported.8–9 The LATS for nitrate has been traditionally considered constitutive, this hypothesis is supported by membrane depolarization studies.10
Concerning to the regulation of root N uptake, there is a general agreement on the hypothesis that feedback repression exerted by the nitrogen nutritional status of the plant11–24 is involved in the control of NH4+ and NO3− uptake systems.
In contrast to the large number of communications on N uptake kinetics and its regulation in herbaceous species,4,25–34 it only has been reported in spruce,7 Quercus suber35 and Citrus concerning woody species.23,36–39 Complex interactions govern nitrate availability and N demand during root development and concerning root architecture. The discrepancy between field observations and conclusions drawn from physiological approaches probably results from our lack of knowledge on the processes involved and in the regulation of nitrate uptake.
The HATS for NO3− uptake is sensitive to metabolic inhibitors and appears to be an active transport system.5,40–42 The mechanism of energy coupling for active NO3− transport by HATS has been investigated in a limited number of species by means of electrophysiological studies.10,43–44 Nitrate absorption was associated with depolarization of cell membrane electrical potential difference (ΔΨ), which was inducible by NO3− and saturable with respect to exogenous NO3− concentration.10 These observations are consistent with a mechanism for NO3− uptake by the HATS involving a 2H+:1NO3− symport moved by the energy derived from the proton gradient generated by the plasma membrane H+-ATPase.43–45 However, very little information has been reported about the energy dependence for the NO3− transport, especially in fruit trees in which NO3− uptake plays a preponderant role after mineral fertilization, when NO3− levels in soil are raised during a limited period of time.36
The aim of the present report was to improve the general knowledge about NO3− uptake and their regulation in two different Citrus rootstocks widely used in agriculture, We have characterized the nitrate transport system and its regulation in induced and uninduced Citrus rootstocks, namely Troyer citrange (salt sensitive) and Cleopatra mandarin (salt tolerant).46
Material and Methods
Plant material.
Cleopatra mandarin (Citrus reshni) and Troyer citrange (Citrus sinensis L. Osbeck × Poncirus trifoliata Blanco) seeds were germinated under greenhouse conditions in 0.5 L pots filled with fine sand, and irrigated twice a week with distilled water. One-month-old seedlings were watered with N-free Hoagland's solution47 supplemented with 1 mM NH4NO3. Temperatures ranged between 16–18°C (night) and 25–27°C (day). Relative humidity was maintained at approximately 80%. Prior to the experiments, three-month-old Cleopatra mandarin or Troyer citrange seedlings with a single shoot were selected for uniformity of size, de-potted, and transferred for seven days to aerated Hoagland solution 1 mM NH4NO3 on hydroponic culture to level up their nutritional status. To study the NO3− uptake mediated by HATS, seedlings were separated into two different groups, one group was grown in nitrogen-free solution for seven days to make sure only NO3− constitutively expressed transport systems continued working (uninduced seedlings), and the other group was grown in nitrogen-free solution for seven days and induced with 0.2 mM KNO3 for three days (induced seedlings) (based on the results obtained in the regulation section). To study the NO3− mediated by LATS, seedlings were separated into two different groups, one group was grown in nitrogen-free solution for seven days (uninduced seedlings) and the other group was grown in a complete Hoagland's solution for seven days and pre-treated with 3 mM KNO3 for one day (pre-treated seedlings). To maintain similar K+ and Ca2+ concentration in the nutrient solutions, K2SO4 and CaSO4 were added to compensate the absence of 1.5 mM KNO3 and 3 mM Ca(NO3)2 of Hoagland solution. The pH of the nutrient solutions was adjusted to 6.0 with 1 M KOH.
Measurement of NO3− uptake.
Nitrate uptake was determined by placing six seedlings in a pyrex 200 mL glass containing uptake solution (Hoagland solution N-free added with K2SO4 and CaSO4, 1.0 mM MES (pH 6.0) and NaNO3 at concentrations ranging between 0.01 and 10 mM). Solutions were aerated vigorously. Water losses by transpiration and evaporation were compensated by frequent additions of water, maintaining the solution volume approximately constant. Aliquots (0.25–1 mL) were taken at 30 min intervals up to 3–6 h depending on the external NO3− concentration. Uptake rates were determined by measuring the disappearance of NO3− from the uptake solutions as a function of time, calculated by linear regression. Nitrate was determined spectrophotometrically by measuring absorbance at 212 nm.48 The nitrate uptake rate was expressed as µmol NO3− g−1 root fresh weight h−1. Based on our previous results36 NO3− uptake above 1 mM [NO3−]0, measured NO3− uptake appeared to result from the participation of two transportsystems (HATS + LATS). Thus, The NO3− mediated by LATS was calculated by subtracting the measured NO3− uptake at concentrations >1 mM [NO3−]0 and the calculated Vmax for the HATS. External NO3− concentrations of 0.2 mM and 3 mM were selected to assay the activities of both HATS and HATS+LATS, respectively, in the following studies.36
Kinetics of NO3− uptake.
The kinetics of NO3− uptake as a function of [NO3−]0 were measured in uninduced seedlings with [NO3−]0 ranging from 10 µM to 10 mM, in induced seedlings with [NO3−]0 ranging from 10–800 µM and in pre-treated seedlings with [NO3−]0 ranging from 1–10 mM. The double reciprocal plots of the uptake rates versus substrate concentrations were subjected to linear regression analysis. The Michaelis-Menten kinetic constants (Km and Vmax), in both uninduced and induced seedlings, were calculated from these regression equtions in the concentration range of 0.01–1 mM NO3−.49
Regulation of NO3− uptake.
Re-supply experiments were performed to investigate the regulation of NO3− uptake after NO3− starvation. Uninduced seedlings were transferred for eight days into N-free Hoagland solutions supplemented with 0.2 or 3 mM KNO3. These solutions were renewed daily to prevent depletion. Nitrate uptake was measured daily after re-supply at both 0.2 and 3 mM [NO3−]0 in seedlings transferred into solutions 0.2 or 3 mM KNO3, to estimate the activities of both HATS and LATS, respectively.
Metabolic inhibitor studies.
The effect of 2,4-DNP (metabolic inhibitor) and DCCD and DES (plasmalemma H+-ATPase inhibitors) was studied in both uninduced and induced seedlings. The compound 2,4-DNP was added to the uptake solutions at a concentration of 20 µM. For the plasmlemma H+-ATPase assays, the seedlings were preincubated with 0.1 mM of either DCCD or DES for 1 h and were added in the same concentration to the uptake solutions. Nitrate concentration for the uptake solutions was 0.2 mM in both uninduced and induced seedlings or 3 mM in uninduced seedlings. For experiments with DES or DCCD, all solutions (including control solutions) contained 0.25% (v/v) ethanol.
External pH effect.
The effect of pH on NO3− uptake was studied in uninduced seedlings grown hydroponically as described above. Uptake solutions contained 1 mM MES-TRIS (pH 4, 5, 6, 7, 8 or 9) and 0.2 or 3 mM NaNO3. The uptake measurements were carried out as mentioned above.
Results
Kinetics of NO3− uptake.
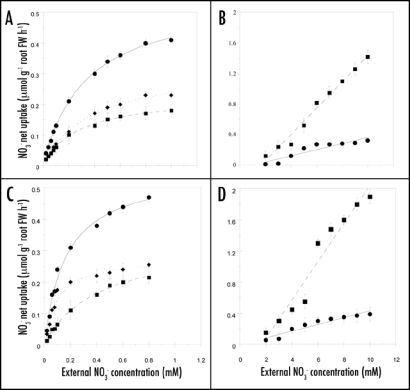
When external NO3− concentrations are below 1 mM, nitrate uptake fits a typical Michaelis-Menten curve, reaching saturation at 0.5 mM external NO3− concentration, typical of the activity of a saturable HATS (Fig. 1A and C), similar nitrate uptake patterns were described previously in uninduced Cleopatra mandarin and Troyer citrange seedlings.36
Figure 1.
Kinetics of NO3− net uptake into Cleopatra mandarine (A and B) and Troyer citrange (C and D) roots in the low [(A–C) • cHATS+iHATS; + iHATS;  cHATS] and high NO3− concentration range (B–D) • LATS of pre-treated seedlings;
cHATS] and high NO3− concentration range (B–D) • LATS of pre-treated seedlings;  LATS of uninduced seedlings). iHATS is estimated by subtracting cHATS from cHATS+iHATS. NO3− net uptake measured above 1 mM external NO3− concentration is considered to be the combined contributions of HATS+LATS. LATS is estimated by subtracting cHATS Vmax from HATS+LATS. Each data point is the mean of 24 replicates with SE values shown as vertical bars.
LATS of uninduced seedlings). iHATS is estimated by subtracting cHATS from cHATS+iHATS. NO3− net uptake measured above 1 mM external NO3− concentration is considered to be the combined contributions of HATS+LATS. LATS is estimated by subtracting cHATS Vmax from HATS+LATS. Each data point is the mean of 24 replicates with SE values shown as vertical bars.
When seedlings were exposed to a temporary deprivation of NO3−, they exhibited lower NO3− uptake rates than seedlings induced by exposure to NO3− after the starvation period (Fig. 1A and C). NO3− uptake was mediated by cHATS when seedlings were exposed to a temporary lack of NO3− (uninduced seedlings) and NO3− uptake was mediated by iHATS + cHATS when seedlings were induced by NO3− (induced seedlings). iHATS is estimated by subtracting cHATS from cHATS+iHATS.
The apparent Km values of the low concentration saturable systems for NO3− uptake in uninduced (cHATS) and induced (iHATS) in Cleopatra mandarin seedlings were 281 ± 8 and 225 ± 10 µM and the corresponding Vmax values were 0.25 ± 0.01 and 0.32 ± 0.02 µmol g−1 root FW h−1, respectively (Fig. 1A). Figure 1C shows similar kinetics constant to Troyer citrange in both cHATS and iHATS transport systems when compared with Cleopatra mandarin. These values were 0.26 ± 0.01 µmol g−1 root FW h−1 for Vmax and 315 ± 12 µM for Km in the cHATS and 0.33 ± 0.01 µmol g−1 root FW h−1 for Vmax and 204 ± 15 µM for Km in the iHATS. Kinetic constant values for HATS to uninduced seedlings for both rootstocks were similar to those shown previously by Cerezo et al.36
In the high concentration range (1–10 mM), NO3− net uptake increased almost linearly with external NO3− concentration in both Cleopatra mandarin and Troyer citrange seedlings (Fig. 1B and D), indicating the action of a non-saturable LATS as it was described by Cerezo et al.36
In this study, after 24 h of exposure with a 3 mM NO3− solution, the uptake rate constants in pre-treated seedlings were 0.040 and 0.044 µmolg−1 root FW h−1 mM−1 for Cleopatra mandarin and Troyer citrange, respectively, which represented a reduction of 77% and 82% with respect to uninduced seedlings. Average value of NO3− uptake (0.25 ± 0.03 µmolg−1 root FW h−1) was near to Vmax in cHATS (Fig. 1B and D), which represents 20% of NO3− net uptake measured at 3 mM external NO3− concentration.
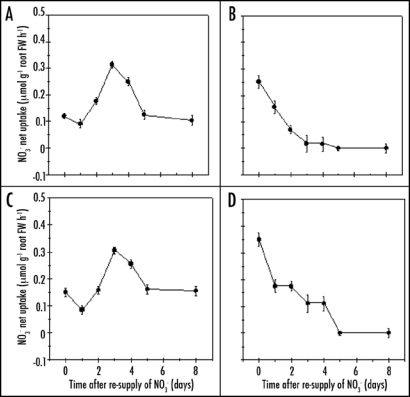
Regulation of NO3− uptake by NO3− resupplies.
To study the regulation of NO3− uptake by NO3− resupplies, Cleopatra mandarin or Troyer citrange uninduced seedlings were transferred to 0.2 mM NO3− solution for 8 days resulted an increased activity of the HATS, where maximum stimulation was reached after 3 days (0.312±0.012 and 0.305 ± 0.010 µmolg−1 root FW h−1 for Cleopatra mandarin and Troyer citrange, respectively). Stimulation then decreased to a minimum of NO3− uptake equal at NO3− uptake in uninduced seedlings (0.103 ± 0.002 and 0.155 ± 0.022 µmol g−1 root FW h−1) for Cleopatra mandarin and Troyer citrange, respectively (Fig. 2A and C).
Figure 2.
Time course of NO3− net uptake in roots of uninduced seedlings of Cleopatra mandarine and Troyer citrange after re-supply of NO3− at 0.2 mM (A and C, respectively) or 3 mM (B and D, respectively). NO3− net uptake was measured at both 0.2 (iHATS) and 3 mM NO3− (cHATS+LATS). The value calculated of LATS only in Cleopatra mandarine and Troyer citrange (B and D, respectively) was obtained by subtracting the values of Vmax of each rootstock from HATS+LATS (data not shown). Each data point is the average of 18 replicates with SE values shown as vertical bars.
The opposite response was observed for the calculated LATS-mediated NO3− uptake in both Cleopatra mandarin and Troyer citrange seedlings, which decreased upon transfer of the uninduced seedlings to N-free Hoagland solutions supplemented with 3 mM KNO3 (Fig. 2B and D). The inhibition of the LATS activity by NO3− re-supply was 30% in both rootstocks after one day. After three days, NO3− uptake was similar to Vmax in both rootstocks, 0.25 ± 0.01 to Cleopatra mandarin (Fig. 1A) and 0.26 ± 0.01 µmol g−1 root FW h−1 to Troyer citrange (Fig. 1C).
LATS values (Fig. 2B and D) were calculated by subtracting the values of cHATS Vmax of each rootstock from HATS + LATS. The NO3− uptake observed to [NO3−]0 3 mM after three days of resupply of 3 mM KNO3 were reduced in both rootstocks. The values were lower than 0.12 and 0.30 µmol g−1 root FW h−1 to Cleopatra mandarin and Troyer citrange, observed to 0.2 mM [NO3]0 after three days of resupply of 0.2 mM KNO3 (Fig. 2A and C), which indicates that iHATS is actively inhibited in the presence of NO3− to 3 mM.
Effect of metabolic inhibitors on NO3− uptake.
To study the effect of metabolic inhibitors on NO3− uptake, an ATP-synthesis inhibitor (2,4-DNP) and two plasmalemma ATPase inhibitors (DCCD and DES) were used. The effect of these compounds was tested in uninduced and induced Cleopatra mandarin and citrange Troyer seedlings. The presence of 2,4-DNP prevented the uptake of NO3− by cHATS, iHATS and LATS from 50–98%. In the same conditions, treatment of roots with either DCCD or DES, which have been shown to inhibit the plasmalemma H+-ATPase, also inhibited root nitrate absorption on all transport systems from 48%–85% (Tables 1 and 2).
Table 1.
Effect of inhibitors on NO3− net uptake into Cleopatre mandarine roots
| cHATS | iHATS | LATS | ||||
| Treatment | NO3− net uptake | % inhibition | NO3− net uptake | % inhibition | NO3− net uptake | % inhibition |
| Control | 0.103±0.010 | 0.197±0.012 | 0.417±0.030 | |||
| 2,4-DNP | 0.051±0.001* | 50 | 0.003±0.001* | 98 | 0.109±0.011* | 74 |
| DCCD | 0.036±0.002* | 65 | 0.056±0.003* | 72 | 0.148±0.012* | 64 |
| DES | 0.031±0.012* | 70 | 0.043±0.002* | 78 | 0.217±0.020* | 48 |
The cHATS, iHATS and cHATS+LATS activities were measured in 0.2 mM NO3−, uninduced seedlings, 0.2 mM NO3−, induced seedlings by 3 d, and 3 mM NO3−, uninduced seedlings, respectively. iHATS and LATS were calculated by subtracting the NO3− net uptake measured at 0.2 mM NO3− in uninduced seedlings from these measured at 0.2 mM NO3− in induced seedlings and, 3 mM NO3− in uninduced seedlings, respectively. Each data point is the average of 24 replicates with ± SE.
Significant at 5% level respect to the control. NS not significant. NO3− net uptake is expressed as µmol g−1 root FW h−1.
Table 2.
Effect of inhibitors on NO3− net uptake into Troyer citrange roots
| cHATS | iHATS | LATS | ||||
| Treatment | NO3− net uptake | %inhibition | NO3− net uptake | % inhibition | NO3− net uptake | % inhibition |
| Control | 0.098 ± 0.010 | 0.127 ± 0.012 | 0.386 ± 0.030 | |||
| 2,4-DNP | 0.046 ± 0.001* | 53 | 0.002 ± 0.001* | 98 | 0.116 ± 0.011* | 70 |
| DCCD | 0.031 ± 0.002* | 68 | 0.038 ± 0.003* | 70 | 0.154 ± 0.012* | 60 |
| DES | 0.027 ± 0.012* | 72 | 0.038 ± 0.002* | 70 | 0.193 ± 0.020* | 50 |
The cHATS, iHATS and cHATS+LATS activities were measured in 0.2 mM NO3−, uninduced seedlings, 0.2 mM NO3−, induced seedlings by 3 d, and 3 mM NO3−, uninduced seedlings, respectively. iHATS and LATS were calculated by subtracting the NO3− net uptake measured at 0.2 mM NO3− in uninduced seedlings from these measured at 0.2 mM NO3− in induced seedlings and, 3 mM NO3− in uninduced seedlings, respectively. Each data point is the average of 24 replicates with ± SE.
Significant at 5% level respect to the control. NS not significant. NO3− net uptake is expressed as µmol g−1 root FW h−1.
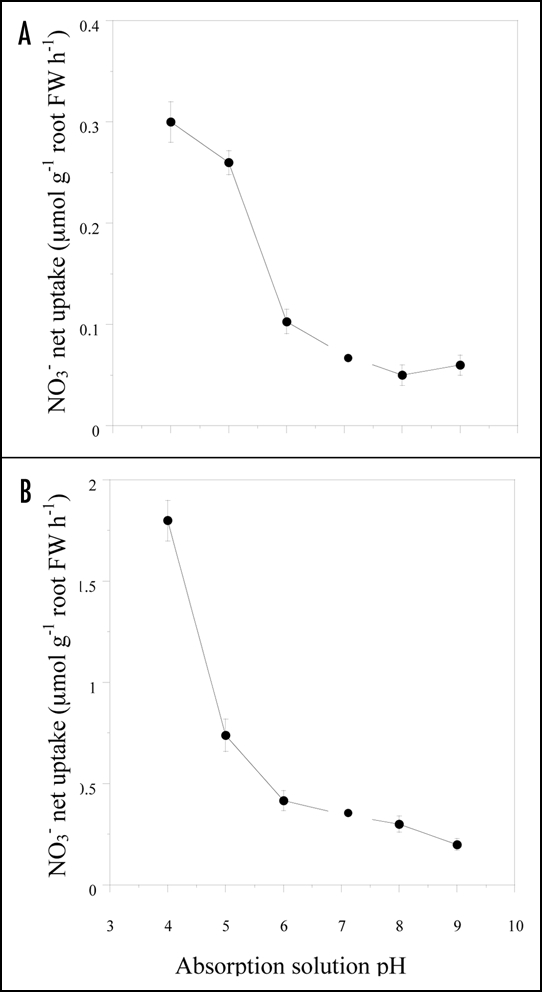
Effect of external pH on NO3− uptake.
Both the HATS-mediated and the LATS-mediated NO3− uptake in uninduced Cleopatra mandarin seedlings displayed a strong dependence upon external pH, with a marked optimum in the acidic range. The NO3− uptake at both 0.2 mM and 3 mM [NO3−]0 were markedly inhibited (by more than 75%) when the solution pH was raised from pH 4 to 7 (Fig. 3A and B). Similar results were found in Troyer citrange (24). These results showed that the uptake rate was reduced approximately by 70% in both HATS and LATS transport systems, when the solution pH was raised from pH 4 to 7.
Figure 3.
Effect of the pH of the absorption solution on NO3− net uptake into roots of Cleopatra mandarine seedlings. For the cHATS (A), NO3− net uptake was measured at 0.2 mM NO3− in uninduced seedlings. For the HATS+LATS, NO3− net uptake was measured at 3 mM NO3− in uninduced seedlings. The value of LATS only (B) was obtained by subtracting the values of HATS from HATS+LATS (data not shown) for each treatment. Each data point is the average of 18 replicates with SE values shown as vertical bars.
Discussion
Nitrate uptake in Troyer citrange and Cleopatra mandarin is controlled by three transport systems. Two of them are HATS, cHATS and iHATS, that show Michaelis-Menten kinetics, the third is a LATS that is a linear non-saturable system. cHATS is constitutive and independent of N-status. In Citrus, the role of cHATS is probably to enable the cytoplasmic concentration of nitrate to rise to a level sufficient for the induction of the higher capacity iHATS as described before in barley by Behl et al.50
For the regulation of iHATS, this system is under feedback regulation by the N-status of the plant, being upregulated after 1–3 d of NO3− exposure, and downregulated between 3–8 d of NO3− exposure. Moreover, our data provide evidence that in both rootstocks, the LATS is repressed by NO3−.
Our results show that the activities of the three transport systems (cHATS, iHATS and LATS) are differently affected either by the N-status of the seedlings or by NO3− itself (Fig. 2). In other plants,5,51–53 a period of induction of several hours was usually required before the effect of resupplying NO3− became apparent on cHATS. However, in both Cleopatra mandarin and Troyer citrange, the time of exposure to NO3− required for induction was unusually long, since up to three days were necessary for maximal response. This is coincidental with the slow induction observed in other woody species such as spruce.7 Moreover, the inductive enhancement factor for Vmax was lower than that observed in other plants in which flux increases at least 5 to 30 times.5–7 These results suggest that in Citrus, the iHATS activity is NO3− upregulated and N-downregulated, whereas cHATS is not affected by NO3− exposure.
Very little information exists about the LATS. Several reports indicate that different LATS are regulated differently by N present in plants.54–55 Different authors have shown that the LATS is constitutive and does not require induction by nitrate, as it is shown by both kinetic5 and electrophysiological10 studies, but this does not mean that the LATS is not under-regulation, because it is influenced by N demand.25 We found that after resupply, NO3− uptake mediated by LATS decreased in Cleopatra mandarin and Troyer citrange seedlings, indicating that LATS is repressed in plants under high N status. These results suggest that LATS is N-repressive and does not work in plants pre-treated with 3 mM NO3− according to results shown previously by Cerezo et al.22 This effect could be explained by a N-downregulation of the LATS by NO3− itself or by amino acids.14,56–58
In some species HATS for NO3− uptake is sensitive to metabolic inhibitors and appears to be an active transport system5,4 based on electrophysiological studies.10,43–44 These studies are consistent with a mechanism for NO3− uptake by the HATS involving a 2H+:1NO3− symport moved by the energy derived from the proton gradient generated by the plasma membrane H+-ATPase.10,43–45
Results in both Cleopatra mandarin and Troyer citrange seedlings support this proposal since both cHATS and iHATS were inhibited by a metabolic uncoupler (2,4-DNP) and by inhibitors of H+ translocation ATPases (DCCD and DES) (Tables 1 and 2). This is further supported by the pH response of the NO3− transport system, which was inhibited at alkaline pH values (Fig. 3A). The observation of an acidic pH optimum coincides with previous studies of the pH dependence of nitrate uptake.44
The LATS was characterized as constitutive5,10 and relatively insensitive to metabolic inhibition. However, considering the determinations of root cytoplasmic NO3− concentrations56 and the transmembrane electrical potential differences measured by Glass et al.10 in barley root cells, it is very unlikely that passive influx of NO3− occurs via the LATS. Glass et al.10 suggested that this system is thermodynamically active and capable of transporting NO3− against its electrochemical potential gradient. These authors also presented evidence indicating that the LATS for NO3− uptake is probably mediated by an electrogenic proton cotransport system. Our results are consistent with this hypothesis, we found that LATS for NO3− uptake in Cleopatra mandarin and Troyer citrange seedlings roots was also inhibited by alkaline external pH (Fig. 3B) and appeared to be very sensitive to metabolic uncouplers (2,4-DNP) and inhibitors of H+ translocation ATPases (DCCD and DES). These results support the possibility that LATS is an active transport system, linked to the H+-ATPase. All systems are carrier-mediated, probably by an electrogenic proton cotransport system.
In unfertilised soil, cultivated areas usually maintain a concentration of NO3− in soil below 1 mM depending on soil moisture and other factors. Taking all together we propose that under these field conditions, the HATS would play a major role in NO3− nutrition and the induction of a nitrate uptake system. Under low rising nitrate concentrations, nitrate uptake takes place through a cHATS, which seems to function as a sensing mechanism for nitrate in the environment of the roots that activates a system with increased inducible capacity (iHATS). After fertilization, when the NO3− level in soil is considerably raised, the LATS enables the plant to absorb large amounts of NO3− in a limited period of time, which are probably accumulated through different reserve N chemical forms in leaves or other N-storage organs.
A major challenge for the future will be to discover the function of the regulatory mechanism by which the plant monitors its internal status and transduces the signals to modulate the expression and/or activity of the net nitrate transports.
Acknowledgements
Research was supported by Plan de Promoción de la investigación Fundació Caixa-Castelló-UJI 2001-2003, Sindicato de riegos de la Diputación de Castellón, the Ministerio de Ciencia y Tecnología (BFI2003-06948) and the Ministerio de Educación, Cultura y Deporte (Grant AP2002-3620).
Abbreviations
- DCCD
(N-N′-Dicyclohexyl-carbodiimide)
- DES
(Diethylstilbestrol)
- 2,4-DNP
(2,4-dinitrophenol)
- Km
the external ion concentration giving half of the maximum rate (µM)
- MES
(2-[N-Morpholino]ethane-sulfonic acid)
- [NO3−]0
external nitrate concentration
- TRIS
(Tris(hydroxymethyl)-aminomethane)
- Vmax
the calculated maximum rate of ion influx (µmol NO3− g−1 root fresh weight h−1)
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4578
References
- 1.Bush DR. Proton-coupled sugar and amino acid transporter in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1993;44:513–542. [Google Scholar]
- 2.Chapin FS, IIIrd, Moilanen L, Kielland K. Preferential use of organic nitrogen for growth by a non-mycorrhizal artic sedge. Nature. 1993;361:150–153. [Google Scholar]
- 3.Crawford NM, Glass ADM. Molecular and physiological aspects of nitrate uptake in plants. Trends in Plant Sciences. 1998;3:389–395. [Google Scholar]
- 4.Forde BG, Clarkson DT. Nitrate and ammonium nutrition of plants: Physiological and molecular perspectives. Adv Bot Res. 1999;30:1–90. [Google Scholar]
- 5.Siddiqi MY, Glass ADM, Ruth TJ, Rufty TW. Studies of the uptake of nitrate in barley. I. Kinetics of 13NO3− influx. Plant Physiol. 1990;93:1427–1432. doi: 10.1104/pp.93.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aslam M, Travis RL, Huffaker RC. Comparative kinetics and reciprocal inhibition of nitrate and nitrite uptake in roots of uninduced and induced barley (Hordeum vulgare L.) seedlings. Plant Physiol. 1992;99:1124–1133. doi: 10.1104/pp.99.3.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kronzucker HJ, Siddiqi MY, Glass ADM. Kinetics of NO3− influx in spruce. Plant Physiol. 1995;109:319–326. doi: 10.1104/pp.109.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang NC, Chiang CS, Crawford NM, Tsay YF. CHL1 encodes a component of the low-affinity nitrate uptake system in Arabidopsis and shows cell type-specific expression in roots. Plant Cell. 1996;8:2183–2191. doi: 10.1105/tpc.8.12.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou JJ, Theodoulou FL, Muldin I, Ingemarsson B, Miller AJ. Cloning and functional characterization of Brassica napus transporter that is able to transport nitrate and histidine. J Biol Chem. 1998;273:12017–12023. doi: 10.1074/jbc.273.20.12017. [DOI] [PubMed] [Google Scholar]
- 10.Glass ADM, Shaff JE, Kochian LV. Studies of the uptake of nitrate in barley. IV. Electrophysiology. Plant Physiol. 1992;99:456–463. doi: 10.1104/pp.99.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ullrich WR, Larsson M, Larsson CM, Lesch S, Novacky A. Ammonium uptake in Lemna gibba G1, Related membrane potential changes, and inhibition of anion uptake. Physiol Plantarum. 1984;61:369–376. [Google Scholar]
- 12.Morgan MA, Jackson WA. Suppression of ammonium uptake by nitrogen supply and its relief during nitrogen limitation. Physiol Plantarum. 1988;73:38–45. [Google Scholar]
- 13.Clarkson DT, Lüttge U. Inducible and repressible nutrient transport systems. Progress Bot. 1991;52:61–83. [Google Scholar]
- 14.Lee RB, Purves J, Ratcliffe R, Saker L. Nitrogen assimilation and the control of ammonium and nitrate absorption by maize roots. J Exp Bot. 1992;18:1385–1396. [Google Scholar]
- 15.Wang MY, Siddiqi MY, Ruth TJ, Glass ADM. Ammonium uptake by rice roots. I. Fluxes and subcellular-distribution of 13NH4+ Plant Physiol. 1993;103:1249–1258. doi: 10.1104/pp.103.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang MY, Siddiqi MY, Ruth TJ, Glass ADM. Ammonium uptake by rice roots. II. Kinetics of 13NH4+: Influx across the plasmalemma. Plant Physiol. 1993;103:1259–1267. doi: 10.1104/pp.103.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang MY, Glass ADM, Shaff JE, Kochian LV. Ammonium uptake by rice roots. III. Electrophysiology. Plant Physiol. 1994;104:899–906. doi: 10.1104/pp.104.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kronzucker HJ, Siddiqi MY, Glass ADM. Kinetics of NH4+ influx in spruce. Plant Physiol. 1996;110:773–779. doi: 10.1104/pp.110.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Wirén N, Bergfeld A, Ninnemann O, Fromer WB. OsAMT1-1: A high-affinity ammonium transporter from rice (Oryza sativa cv. Nipponbare) Plant Mol Biol. 1997;3:681. [Google Scholar]
- 20.von Wirén N, Gazzarrini S, Gojon A, Frommer WB. The molecular physiology of ammonium uptake and retrieval. Curr Opin Plant Biol. 2000;3:254–261. [PubMed] [Google Scholar]
- 21.Gazzarrini S, Lejay T, Gojon A, Ninnemann O, Frommer WB, von Wirén N. Three functional transporters for constitutive, diurnally regulated, and starvation-induced uptake of ammonium into Arabidopsis roots. Plant Cell. 1999;11:937–947. doi: 10.1105/tpc.11.5.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerezo M, Tillard P, Gojon A, Primo-Millo E, Garcia-Agustin P. Characterization and regulation of ammonium transport systems in Citrus plants. Planta. 2001;214:97–105. doi: 10.1007/s004250100590. [DOI] [PubMed] [Google Scholar]
- 23.Pal'ove-Balang P, Mistrik I. Differential effect of amino acids on nitrate uptake by intact maize roots. Biologia. 2002;57:119–124. [Google Scholar]
- 24.Loqué D, von Wirén N. Regulatory levels for the transport of ammonium in plant roots. J Exp Bot. 2004;55:1293–1305. doi: 10.1093/jxb/erh147. [DOI] [PubMed] [Google Scholar]
- 25.Touraine B, Glass ADM. NO3− and ClO3− fluxes in the chl1-5 mutant of Arabidopsis thaliana: Does the CHL1-5 gene encode a low-affinity NO3− transporter? Plant Physiol. 1997;114:137–144. doi: 10.1104/pp.114.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daniel-Vedele F, Filleur S, Caboche M. Nitrate transport: A key step in nitrate assimilation. Curr Opin Plant Biol. 1998;1:235–239. doi: 10.1016/s1369-5266(98)80110-6. [DOI] [PubMed] [Google Scholar]
- 27.Forde BG. Nitrate transporters in plants: Structure, function and regulation. Biochimica et Biophysica Acta. 2000;1465:219–235. doi: 10.1016/s0005-2736(00)00140-1. (Fre). [DOI] [PubMed] [Google Scholar]
- 28.Cerezo M, Tillard P, Filleur S, Muñoz S, Daniel-Vedele F, Gojon A. Major alterations of the regulation of root NO3− uptake are associated with the mutation of Nrt2.1 and Nrt2.2 genes in Arabidopsis. Plant Physiol. 2001;127:262–271. doi: 10.1104/pp.127.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filleur S, Dorbe MF, Cerezo M, Orsel M, Granier F, Gojon A, Daniel-Vedele F. An Arabidopsis T-DNA mutant affected in Nrt2 genes is impaired in nitrate uptake. FEBS Letters. 2001;489:220–224. doi: 10.1016/s0014-5793(01)02096-8. [DOI] [PubMed] [Google Scholar]
- 30.Touraine B, Daniel-vedele F, Forde BG. Nitrate uptake and its regulation. In: Lea PJ, Morot-Gaudry JF, editors. Plant nitrogen. Berlin-Heidelberg: INRA Editions and Springer-Verlag; 2001. pp. 1–36. [Google Scholar]
- 31.Williams LE, Miller AJ. Transporters responsible for the uptake and partitioning of nitrogenous solutes. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:659–688. doi: 10.1146/annurev.arplant.52.1.659. [DOI] [PubMed] [Google Scholar]
- 32.Glass ADM, Britto DT, Kaiser BN, Kinghorn JR, Kronzucker HJ, Kumar A, Okamoto M, Rawat S, Siddiqi MY, Unkles SE, Vidmar J. The regulation of nitrate and ammonium transport systems in plants. J Exp Bot. 2002;370:855–864. doi: 10.1093/jexbot/53.370.855. [DOI] [PubMed] [Google Scholar]
- 33.Lejay L, Gansel X, Cerezo M, Tillard P, Müller C, Krapp A, vonWirén N, Daniel-Vedele F, Gojon A. Regulation of root ion transporters by photosynthesis: Functional importance and relation with hexokinase. Plant Cell. 2003;15:2218–2232. doi: 10.1105/tpc.013516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nazoa P, Vidmar JJ, Tranbarger TJ, Mouline K, Damiani I, Tillard P, Zhuo D, Glass ADM, Touraine B. Regulation of the nitrate transporter gene AtNRT2.1 in Arabidopsis thaliana: Responses to nitrate, amino acids and developmental stage. Plant Molecular Biol. 2003;52:689–703. doi: 10.1023/a:1024899808018. [DOI] [PubMed] [Google Scholar]
- 35.Mata C, van Vemde N, Clarkson DT, Martins-Louçao MA, Lambers H. Influx, efflux and net uptake of nitrate in Quercus suber seedlings. Plant and Soil. 2000;221:25–32. [Google Scholar]
- 36.Cerezo M, García-Agustín P, Serna MD, Primo-Millo E. Kinetics of nitrate uptake by Citrus seedlings and inhibitory effects of salinity. Plant Science. 1997;126:105–112. [Google Scholar]
- 37.Serna MD, Borras R, Legaz F, Primo-Millo E. The influence of nitrogen concentration and ammonium/nitrate ratio on N-uptake, mineral composition and yield of citrus. Plant Soil. 1992;147:13–23. [Google Scholar]
- 38.Cerezo M, Flors V, Legaz F, García-Agustín P. Characterization of the low affinity transport system for NO3− uptake by Citrus roots. Plant Sci. 2000;160:95–104. doi: 10.1016/s0168-9452(00)00363-0. [DOI] [PubMed] [Google Scholar]
- 39.Sorgonà A, Abenavoli MR, Cacco G. A comparative study between two citrus rootstocks: Effect of nitrate on the root morpho-topology and net nitrate uptake. Plant and Soil. 2005;270:257–267. [Google Scholar]
- 40.Jackson WA, Flescher D, Hageman RH. Nitrate uptake by dark-grown corn seedlings: Some characteristics of apparent induction. Plant Physiol. 1973;51:120–127. doi: 10.1104/pp.51.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao KP, Rains DW. Nitrate absorption by barley. I. Kinetics and energetics. Plant Physiol. 1976;57:55–58. doi: 10.1104/pp.57.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glass ADM, Siddiqi MY, Ruth TJ, Rufty TW. Studies of the uptake of nitrate in barley. II. Energetics. Plant Physiol. 1990;93:1585–1589. doi: 10.1104/pp.93.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ullrich WR, Novacky A. Nitrate-dependent membrane potential changes and their induction in Lemna gibba. Plant Sci Lett. 1981;22:211–217. [Google Scholar]
- 44.McClure PR, Kochian LV, Spanswick RM, Shaff JE. Evidence for cotransport of nitrate and protons in maize roots. II. Measurement of NO3− and H+ fluxes with ion-selective microelectrodes. Plant Physiol. 1990;93:290–294. doi: 10.1104/pp.93.1.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santi S, Locci G, Pinton R, Cesco S, Varanini Z. Plasma membrane H+-ATPase in maize roots induced for NO3− uptake. Plant Physiol. 1995;109:1277–1283. doi: 10.1104/pp.109.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romero-Aranda R, Moya JL, Tadeo FR, Legaz F, Primo-Millo E, Talon M. Physiological and anatomical disturbances induced by chloride salts in sensitive and tolerant citrus: Beneficial and detrimental effects of cations. Plant Cell and Environ. 1998;21:1243–1253. [Google Scholar]
- 47.Hoagland DR, Arnon DJ. The water culture method for growing plants without soil. Califor Agric Exp Stat Cir. 1950;347:1–32. [Google Scholar]
- 48.APHA, AWWA, WPCF, author. Standard methods for the examination of wastewater. 15th ed. Public Edit. 1980. p. 1134. [Google Scholar]
- 49.Segel IH. Enzyme kinetics. New York: John Wiley and Jons; 1975. Behaviour and analysis of rapid equilibrium and steady state enzyme systems; pp. 100–160. [Google Scholar]
- 50.Behl R, Tischnner R, Raschke K. Induction of a high-capacity nitrate-uptake mechanism in barley roots prompted by nitrate uptake through a constitutive low-capacity mechanism. Planta. 1998;176:235–240. doi: 10.1007/BF00392450. [DOI] [PubMed] [Google Scholar]
- 51.Agüera E, de la Haba P, Fontes AG, Maldonado JM. Nitrate and nitrite uptake and reduction by intact sunflower plants. Planta. 1990;182:149–154. doi: 10.1007/BF00239997. [DOI] [PubMed] [Google Scholar]
- 52.Hole DJ, Emran AM, Fares Y, Drew MC. Induction of nitrate transport in maize roots, and kinetics of influx, measured with nitrogen-13. Plant Physiol. 1990;93:642–647. doi: 10.1104/pp.93.2.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Botella MA, Cerdá A, Lips SH. Kinetics of NO3− and NH4+ uptake by wheat seedlings: Effect of salinity and nitrogen source. J Plant Physiol. 1994;144:53–57. [Google Scholar]
- 54.Lauter FR, Ninnemann O, Bucher M, Riesmeier JW, Frommer WB. Preferential expression of an ammonium transporter and of two putative nitrate transporters in root hairs of tomato. Proc Natl Acad Sci. 1996;93:8139–8144. doi: 10.1073/pnas.93.15.8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lejay L, Tillard P, Lepetit M, Olive FD, Filleur S, Daniel-Vedele F, Gojon A. Molecular and functional regulation of two NO3− uptake systems by N- and C-status of Arabidopsis plants. The Plant J. 1999;18:1–11. doi: 10.1046/j.1365-313x.1999.00480.x. [DOI] [PubMed] [Google Scholar]
- 56.King BJ, Siddiqi MY, Glass ADM. Studies of the uptake of nitrate in barley. V. Estimation of root cytoplasmic nitrate concentration using nitrate reductase activity - Implications for nitrate influx. Plant Physiol. 1992;99:1582–1589. doi: 10.1104/pp.99.4.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rufty TW, Israel DW, Volk RJ, Qiu J, Sa T. Phosphate regulation of nitrate assimilation in soybean. J Exp Bot. 1993;44:879–891. [Google Scholar]
- 58.Imsande J, Tourraine B. N demand and the regulation of nitrate uptake. Plant Physiol. 1994;105:3–7. doi: 10.1104/pp.105.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]