Abstract
Among the various rhizospheric interactions, plant root-microbe interactions are very important both economically and ecologically. The interaction of plant roots with plant growth promoting rhizobacteria (PGPR) have been studied in case of symbiotic organisms. However, the knowledge on interaction with other PGPRs such as biocontrol Bacillus sps. is vastly unexplored. Especially the complex root surface chemistry and its effect on modulating the bacterial growth and association with the root system has not been investigated. Recently, by adopting a systematic stepwise experimental approach we unraveled the importance of root plane chemistry on the colonization and biofilm formation by B. subtilis, an important biocontrol-PGPR. This study may further increase our understanding in the field of rhizosphere biology and area of root secretions and their possible role in plant microbe interactions.
Key Words: PGPR, biofilm, catechol and ROS
Rhizosphere, the region around the roots harbor wide array of microbial populations, which may be beneficial, neutral or detrimental to plant growth. The reason for this efficient colonization and the presence of increased microbial populations has been ascribed to the nutrient rich environment of the rhizosphere. It has been reported that nearly 40% of total plant photosynthates are secreted through root exudates.1 The studies appreciating the role of root exudates in rhizospheric interactions have been beginning to appear. Among the different groups of microbes which colonize the rhizosphere and the root surface, the plant growth promoting rhizobacteria (PGPR) are a class, which promotes plant growth.2 The plant growth promotion by such PGPRs is primarily rendered by their ability to produce phytohormones, improve the nutrient uptake and protection from pathogenic microorganisms.3 The mechanism by which the biocontrol-PGPR, Bacillus subtilis protects plant roots from pathogenic bacteria include biofilm formation in addition to antibiotic and surfactin production.4,5 Biofilms are structured community of microbial cells encased in a self-produced polymeric matrix.6 Recent research studies have begun to understand and elucidate the genetic pathways controlling the B. subtilis biofilm formation.7–15 No previous study has attempted the question whether, a PGPR, B. subtilis is recognized like pathogen through the well-studied pathogenesis/disease resistance pathways? We employed various plant disease resistance pathway mutants and a transgenic line to determine whether A. thaliana can distinguish between a PGPR, such as B. subtilis, and a plant pathogenic bacterium. In contrast to our initial speculation, this screening step showed that B. subtilis is not recognized as a pathogen by A. thaliana roots as it could colonize the roots of all the plant disease pathway mutants studied. This result though not fully conclusive indicated that A. thaliana roots interact with the B. subtilis through a pathway other than the regular pathogenesis/disease resistance pathways. This forms an important clue for the future studies involving the A. thaliana-B. subtilis interactions. However, we observed the non-colonization and suppression of B. subtilis biofilm formation on the roots of A. thaliana line NahG (Fig. 1), a transgenic line-containing gene for salicylate hydroxylase, which hydrolyzes salicylic acid and results in the overproduction of catechol.16 This suggested that the catechol might be playing a key role in inhibiting the B. subtilis colonization and biofilm formation on the NahG root surface. This speculation was further tested by studying the effect of catechol on in vitro biofilm formation on the abiotic surface and in vivo on the wild type Col-0 plants which showed the suppression of biofilm formation under both the conditions. There is a published evidence that showed that A. thaliana non-host resistance is compromised in NahG plants in response to Pseudomonas syringae pv. phaseolicola 3121.16 However our results showed that the catechol acts on B. subtilis through a pathway, which is altogether different from the mechanism reported for a pathogenic interaction.16
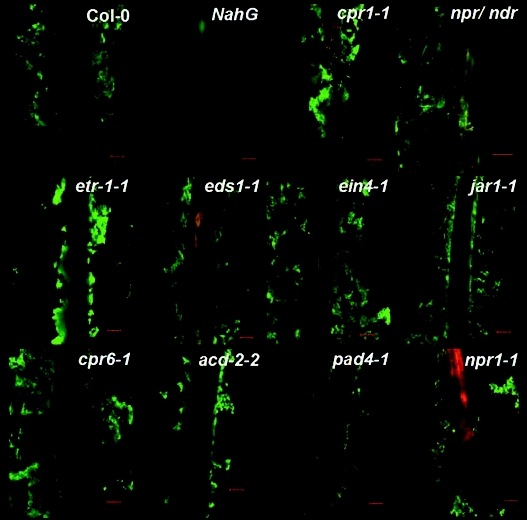
Figure 1.
Confocal microscopy images of Arabidopsis roots co-cultivated with B. subtilis strain FB-17 showing dense biofilm formation on the surface of Col-0, cpr6-1, etr1-1, jar1-1, ein4-1, less biofilm formation on cpr1-1 pad4-1, eds1-1, npr1-1, npr/ndr double mutant compared to the NahG roots.
The lack of biofilm formation on the NahG root surface and a threefold reduction in the B. subtilis cell surface adhesion led us to further speculate that the additional inhibition might occur directly on the root surface through a specific catechol induced biochemical changes. Catechol is a phenolic compound and such compounds are known to generate reactive oxygen species (ROS),17 we hypothesized that the inhibition might be brought about by the higher titers of ROS generated by increased concentrations of catechol on NahG roots. In accordance with our hypothesis the NahG root surface stained and imaged for ROS showed higher ROS generation when compared to all other mutants and wild type Col-0.18 Similarly, a significantly higher titres of surface and exuded ROS production was observed in NahG when compared to Col-0. However, the role of ROS was further conclusively established when the biofilm formation was restored in the NahG roots treated with ascorbic acid, a ROS quencher. Further, we hypothesized that the suppressed binding of B. subtilis due to altered root surface chemistry, might be occurring through the direct suppression of transcriptional operons required for biofilm formation in B. subtilis. In consistent with all the previous results direct catechol treatment resulted in a significant reduction in the transcription levels of the operons yqxM and epsA which are required for biofilm formation in B. subtilis. Finally, with these transcriptional profiling studies, we showed that the suppression of B. subtilis biofilm formation on NahG root surface is due to the presence of catechol on the NahG root surface (and in the surrounding area), resulting in ROS mediated down regulation of genes required for biofilm formation in Bacillus subtilis.
Our findings established the importance of root surface chemistry and secretions in colonization and biofilm formation of B. subtilis. There is a possibility that the biocontrol mechanism driven by biofilm formation is regulated by in planta redox potential in the rhizosphere. It is clear, however, that additional information is required to further clarify the role of gene expression in biofilm formation and efficacy. Studies in rice and Arabidopsis systems have demonstrated that higher catechol levels result in the production of superoxides and H2O2 leading to increased ROS generation.16,19 Further mechanistic studies are required to elucidate the role of catechol and catechol generated ROS in the rhizospheric interactions such as plant-plant and plant-microbe and microbe-microbe interactions. In addition, research studies focusing on the elucidation of novel plant genes that are detrimental in B. subtilis colonization on A. thaliana will shed some more light on this beneficial interaction.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4117
References
- 1.Lynch JM, Whipps JM. Substrate flow in the rhizosphere. In: Keister DL, Cregan B, editors. The rhizosphere and plant growth. Dordrecht: Kluwer Academic; 1991. pp. 15–24. [Google Scholar]
- 2.Kloepper JW, Ryu MN, Zhang S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathol. 2004;94:1259–1266. doi: 10.1094/PHYTO.2004.94.11.1259. [DOI] [PubMed] [Google Scholar]
- 3.Narula N, Deubel A, Gans W, Behl RK, Merbach W. Paranodules and colonization of wheat roots by phytohormone producing bacteria in soil. Plant Soil Environ. 2006;52:119–129. [Google Scholar]
- 4.Bais HP, Fall R, Vivanco JM. Biocontrol of Bacillus subtilis against infection of Arabidopsis root by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol. 2004;134:307–319. doi: 10.1104/pp.103.028712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavaglieri L, Orlando J, Rodriguez MI, Chulze S, Etcheverry M. Biocontrol of Bacillus subtilis against Fusarium verticilloides in vitro and at the maize root level. Res Microbiol. 2005;156:748–754. doi: 10.1016/j.resmic.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: From the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 7.Branda SS, Gonza'lez-Pastor JE, Ben-Yehuda S, Losick R, Kolter R. Fruiting body formation by Bacillus subtilis. Proc Natl Acad Sci USA. 2001;98:11621–11626. doi: 10.1073/pnas.191384198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamon MA, Lazazzera BA. The sporulation transcription factor Spo0A is required for biofilm development in Bacillus subtilis. Mol Microbiol. 2001;42:1199–1209. doi: 10.1046/j.1365-2958.2001.02709.x. [DOI] [PubMed] [Google Scholar]
- 9.Stanley NR, Britton RA, Grossman AD, Lazazzera BA. Identification of catabolite repression as a physiological regulator of biofilm formation by Bacillus subtilis by use of DNA microarrays. J Bacteriol. 2003;185:1951–1957. doi: 10.1128/JB.185.6.1951-1957.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Branda SS, Gonzalez-Pastor JE, Dervyn E, Ehrlich SD, Losick R, Kolter R. Genes involved in formation of structured multicellular communities by Bacillus subtilis. J Bacteriol. 2004;186:3970–3979. doi: 10.1128/JB.186.12.3970-3979.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Branda SS, Chu F, Kearns DB, Losick R, Kolter R. A major protein component of the Bacillus subtilis biofilm matrix. Mol Microbiol. 2006;59:1229–1238. doi: 10.1111/j.1365-2958.2005.05020.x. [DOI] [PubMed] [Google Scholar]
- 12.Hamon MA, Stanley NR, Britton RA, Grossman AD, Lazazzera BA. Identification of AbrB-regulated genes involved in biofilm formation by Bacillus subtilis. Mol Microbiol. 2004;52:847–860. doi: 10.1111/j.1365-2958.2004.04023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kearns DB, Chu F, Branda SS, Kolter R, Losick R. A master regulator for biofilm formation by Bacillus subtilis. Mol Microbiol. 2005;55:739–749. doi: 10.1111/j.1365-2958.2004.04440.x. [DOI] [PubMed] [Google Scholar]
- 14.Stanley NR, Lazazzera B. Defining the genetic differences between wild and domestic strains of Bacillus subtilis that affect poly-γ-DL-glutamic acid production and biofilm formation. Mol Microbiol. 2005;57:1143–1158. doi: 10.1111/j.1365-2958.2005.04746.x. [DOI] [PubMed] [Google Scholar]
- 15.Chu F, Kearns DB, Branda SS, Kolter R, Losick R. Targets of the master regulator of biofilm formation in Bacillus subtilis. Mol Microbiol. 2006;59:1216–1228. doi: 10.1111/j.1365-2958.2005.05019.x. [DOI] [PubMed] [Google Scholar]
- 16.van Wees SCM, Glazebrook J. Loss of non-host resistance of Arabidopsis NahG to Pseudomonas syringae pv. Phaseolicola is due to degradation product of salicylic acid. Plant J. 2003;33:733–742. doi: 10.1046/j.1365-313x.2003.01665.x. [DOI] [PubMed] [Google Scholar]
- 17.Muzandu K, Shaban Z, Ishizuka M, Kazusaka A, Fujita S. Nitric oxide enhances catechol estrogen-induced oxidative stress in LNCaP cells. Free Radic Res. 2005;39:389–398. doi: 10.1080/10715760400029710. [DOI] [PubMed] [Google Scholar]
- 18.Rudrappa T, Quin WJ, Stanley-Wall NR, Bais HP. A degradation product of salicylic acid pathway triggers oxidative stress resulting in the downregulation of Bacillus subtilis biofilm formation on Arabidopsis thaliana roots. Planta. 2007 doi: 10.1007/s00425-007-0480-8. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, Qi M, Mei C. Endogenous salicylic acid protects rice plants from oxidative damage caused by aging as well as biotic and abiotic stress. Plant J. 2004;40:909–919. doi: 10.1111/j.1365-313X.2004.02267.x. [DOI] [PubMed] [Google Scholar]