Abstract
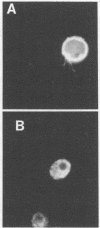
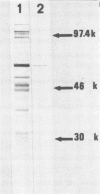
Monoclonal antibodies to Trichomonas vaginalis were prepared by immunizing mice with a cloned isolate of T. vaginalis. Eight antibodies reacted with the same four isolates or strains but did not react with the other T. vaginalis strains or isolates tested. All eight antibodies reacted uniformly with both the body and flagella of T. vaginalis in the immunofluorescence assay but were unreactive by immunoblotting. The antigen(s) recognized by these antibodies was determined to be present on the surface membrane by indirect immunofluorescence assay of live organisms. The antigen(s) was found to be sensitive to periodate oxidation but resistant to pronase digestion. In addition, one monoclonal antibody was derived which reacted with all T. vaginalis isolates or strains tested, as well as with Trichomonas gallinae, Tritrichomonas foetus, and Giardia lamblia. This antibody reacted with the body but not the flagella of Formalin-fixed protozoa in the immunofluorescence assay but failed to react with live organisms. The antigen was found to be periodate resistant but pronase labile. In the immunoblot assay, this antibody detected a single T. vaginalis polypeptide with a molecular weight of 62,000.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F. Antigen analysis of several pathogenic strains of Trichomonas vaginalis. Infect Immun. 1983 Mar;39(3):1041–1047. doi: 10.1128/iai.39.3.1041-1047.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aure J. C., Gjonnaess H. Metronidazole treatment of trichomonal vaginitis. A comparison of cure rates in 1961 and 1967. Acta Obstet Gynecol Scand. 1969;48(3):440–445. doi: 10.3109/00016346909156659. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cooney M. K., Kenny G. E. Immunogenicity of rhinoviruses. Proc Soc Exp Biol Med. 1970 Feb;133(2):645–650. doi: 10.3181/00379727-133-34536. [DOI] [PubMed] [Google Scholar]
- Diamond L. S., Harlow D. R., Cunnick C. C. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72(4):431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- Diddle A. W. Trichomonas vaginalis: resistance to metronidazole. Am J Obstet Gynecol. 1967 Jun 15;98(4):583–585. doi: 10.1016/0002-9378(67)90118-4. [DOI] [PubMed] [Google Scholar]
- Felman Y. M., Nikitas J. A. Trichomoniasis, candidiasis, and Corynebacterium vaginale vaginitis. N Y State J Med. 1979 Sep;79(10):1563–1566. [PubMed] [Google Scholar]
- Honigberg B. M., Livingston M. C., Frost J. K. Pathogenicity of fresh isolates of Trichomonas vaginalis: "the mouse assay" versus clinical and pathologic findings. Acta Cytol. 1966 Sep-Oct;10(5):353–361. [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- KOTT H., ADLER S. A serological study of Trichomonas sp. parasitic in man. Trans R Soc Trop Med Hyg. 1961 Jul;55:333–344. doi: 10.1016/0035-9203(61)90102-x. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lostrom M. E., Stone M. R., Tam M., Burnette W. N., Pinter A., Nowinski R. C. Monoclonal antibodies against murine leukemia viruses: identification of six antigenic determinants on the p 15(E) and gp70 envelope proteins. Virology. 1979 Oct 30;98(2):336–350. doi: 10.1016/0042-6822(79)90557-9. [DOI] [PubMed] [Google Scholar]
- Meingassner J. G., Thurner J. Strain of Trichomonas vaginalis resistant to metronidazole and other 5-nitroimidazoles. Antimicrob Agents Chemother. 1979 Feb;15(2):254–257. doi: 10.1128/aac.15.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E. A. Giardia lamblia: isolation and axenic cultivation. Exp Parasitol. 1976 Feb;39(1):101–105. doi: 10.1016/0014-4894(76)90016-3. [DOI] [PubMed] [Google Scholar]
- Peterson K. M., Alderete J. F. Host plasma proteins on the surface of pathogenic Trichomonas vaginalis. Infect Immun. 1982 Aug;37(2):755–762. doi: 10.1128/iai.37.2.755-762.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock M. E., Bonner S. V. Comparison of undefined medium and its dialyzable fraction for growth of Mycoplasma. J Bacteriol. 1969 Feb;97(2):522–525. doi: 10.1128/jb.97.2.522-525.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein M. F., Chapel T. A. Trichomoniasis, candidiasis, and the minor venereal diseases. Clin Obstet Gynecol. 1975 Mar;18(1):73–88. doi: 10.1097/00003081-197503000-00008. [DOI] [PubMed] [Google Scholar]
- Robinson S. C. Trichomonal Vaginitis Resistant to Metranidazole. Can Med Assoc J. 1962 Apr 7;86(14):665–665. [PMC free article] [PubMed] [Google Scholar]
- Russell W. C., Newman C., Williamson D. H. A simple cytochemical technique for demonstration of DNA in cells infected with mycoplasmas and viruses. Nature. 1975 Feb 6;253(5491):461–462. doi: 10.1038/253461a0. [DOI] [PubMed] [Google Scholar]
- SCHOENHERR K. E. Serologische Untersuchungen über Trichomonaden. Z Immun exp ther. 1956 Apr;113(2):83–94. [PubMed] [Google Scholar]
- Spence M. R., Hollander D. H., Smith J., McCaig L., Sewell D., Brockman M. The clinical and laboratory diagnosis of Trichomonas vaginalis infection. Sex Transm Dis. 1980 Oct-Dec;7(4):168–171. doi: 10.1097/00007435-198010000-00004. [DOI] [PubMed] [Google Scholar]
- Stephens R. S., Tam M. R., Kuo C. C., Nowinski R. C. Monoclonal antibodies to Chlamydia trachomatis: antibody specificities and antigen characterization. J Immunol. 1982 Mar;128(3):1083–1089. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T. Immunologic relationship between genital TRIC, lymphogranuloma venereum, and related organisms in a new microtiter indirect immunofluorescence test. Am J Ophthalmol. 1970 Sep;70(3):367–374. doi: 10.1016/0002-9394(70)90096-6. [DOI] [PubMed] [Google Scholar]