Abstract
It was known that application of glycine betaine (GB) to plants could improve tolerance to stress caused by chilling, frost, salt, drought and high light intensities, and that this effect was accompanied by gene expression changes, but whether the gene expression changes were implicated in GB's effect and which genes were involved has been unclear. In the fourth issue of Physiologia Plantarum, we identified genes upregulated by GB that are involved in reactive oxygen species (ROS) metabolism and membrane trafficking processes. Direct evidence was provided for a role for a membrane trafficking protein (RabA4c) in GB's effect on ROS accumulation during chilling. In this Addendum, we discuss our findings that chilling stress is so closely linked with ROS accumulation. Chilling elevates ROS levels and results in inhibited root growth upon transfer of plants back to normal growing conditions. During the 2–4 day recovery period, ROS levels decline in root tips and in leaves. If ROS accumulation in response to chilling is blocked by pretreatment with GB, optimal root growth begins as soon as plants are transferred back to normal growing conditions without a recovery period, suggesting that chilling stress involves a ROS signaling pathway.
Key Words: chilling, ROS signaling, glycine betaine, membrane trafficking
Chilling stress is a key factor limiting plant survival and distribution on earth with preharvest and post-harvest damage caused by chilling stress resulting in enormous financial losses in agriculture every year. In 1973, Lyons and Raison1 proposed an explanation for the molecular mechanism determining chilling sensitivity based on membrane phase transitions occurring at low temperatures and resulting in destructive events such as membrane damage, ion leakage, impaired photosynthesis and respiration, as well as the production of toxic compounds. This focus on membrane phase transitions led to several studies, examining the role of unsaturated membrane lipids on chilling sensitivity. Among other findings, a correlation was reported between the unsaturation level of membrane lipids and chilling sensitivity in tests of several herbaceous species.2 In addition, it was shown that manipulation of the unsaturation in chloroplast membrane lipids in transgenic plants and blue-green algae had effects on chilling sensitivity. On the other hand, it has also been shown that many chilling resistant plants do not have high levels of unsaturated membrane lipids. In addition, examples are found in Arabidopsis, for example, where mutants having large changes in chloroplast membrane lipids had no change in chilling sensitivity.3 The conclusion, therefore, has been that membrane lipid composition and membrane phase transitions are not the only factors determining chilling sensitivity.
An alternative approach to identifying mechanisms determining chilling sensitivity would be to use chemical genetics, applying a chemical to plants that causes changes in chilling sensitivity by causing changes in gene expression. Attempting to find an experimental system where we could use chemical genetics to study chilling stress, we hypothesized that the beneficial effects on chilling sensitivity caused by treatment of plants with glycine betaine (GB) might be the result of effects on gene expression.4 When we conducted global gene expression studies with microarrays, we did not find GB effects on genes for membrane lipid biosynthesis. Rather, we detected upregulation of genes in leaves5 and roots6 for transcription factors, membrane-trafficking components, ROS-scavenging enzymes and for membrane NADPH-dependent ferric reductases. Genes other than those already reported upregulated by GB and identified from microarray data with confirming Northerns include in roots and leaves a putative bZIP transcription factor At3g62420 and a glutathione S-transferase At1g02930, and in roots aquaporin At5g47450.
To prove that GB's effect depends on gene expression, we went one step further in our studies, showing direct evidence for a role of RabA4c using a knockout mutant. Although Arabidopsis is usually defined as chilling-resistant because it shows no obvious signs of chilling injury, chilling does have an effect because chilled plants show inhibited root growth upon transfer back to normal temperatures. Remarkably, when wild type plants were pretreated with GB, root growth rates after chilling were comparable to nonchilled plants. This could be demonstrated as soon as wild type plants were removed from the chilling chamber by measuring growth rates for roots with movies.7 In contrast to wild type, the RabA4c knockout showed no GB response in the chilling test, proving the requirement for an active RabA4c gene for GB's effect. We have also tested a knockout for the GB upregulated gene for a bZIP transcription factor, and it is similar to the RabA4c knockout in that it shows a lack of a GB response in the chilling test.
Since previous studies had suggested a close linkage between chilling stress and ROS,8 we were interested in investigating ROS in relation to GB treatments and chilling sensitivity. These studies resulted in several lines of evidence directly implicating ROS accumulation with the chilling effects seen in Arabidopsis. Figure 1, for example, shows an experiment analyzing superoxide levels in leaves using NBT staining. Both wild type and knockout plants accumulated superoxide in leaves in response to chilling conditions whereas pretreatment with GB significantly reduced superoxide in wild type while the two knockouts still showed significant staining.
Figure 1.
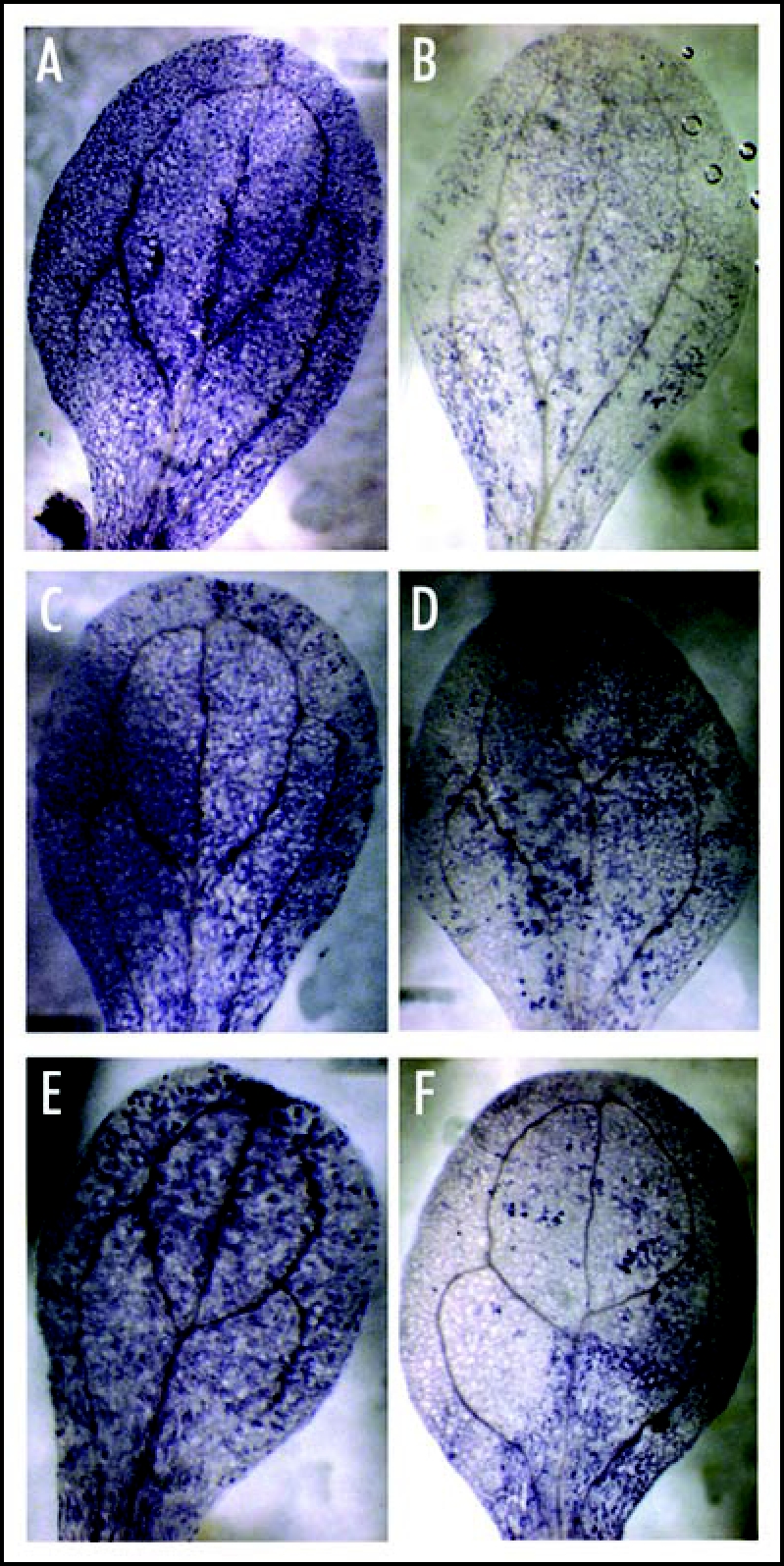
NBT staining for superoxide accumulation in plants subjected to chilling conditions with or without GB pretreatment. Three week old plants of Columbia and the RabA4c and bZIP knockouts were pretreated with water or with GB, then transferred to chilling conditions for two days, after which time superoxide in cotyledonary leaves was visualized by NBT staining. Leaf samples are (A) Columbia without GB pretreatment, (B) Columbia with GB pretreatment, (C) bZIP knockout without GB pretreatment, (D) bZIP knockout with GB pretreatment, (E) RabA4c knockout without GB pretreatment and (F) RabA4c knockout with GB pretreatment.
It is a remarkable observation that GB pretreated plants do not show reduced growth rates after chilling. This observation makes it seem as though chilled plants can avoid the effects of chilling stress as long as they can avoid ROS accumulation. To stimulate further mechanistic studies in relation to the role of ROS in chilling stress, we propose a model (Fig. 2) involving ROS signaling as the molecular mechanism determining chilling sensitivity. According to this model, chilling stress begins with perception of low temperature by an unknown receptor, followed by activation of membrane NADPH oxidase (NOX), causing an increase in ROS levels which signals altered gene expression, resulting in inhibited growth under normal growing conditions in Arabidopsis and in more severe effects in chilling-sensitive plants.
Figure 2.
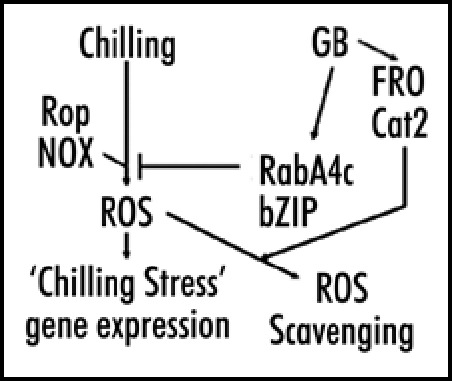
Schematic representation of a model for chilling stress as ROS signaling and gene expression. A competing pathway based on GB action blocks ROS accumulation and alleviates chilling stress.
Given that ROS accumulation via NOX activation plays a central role in the model, the control of NOX activation becomes of special interest. In neutrophils activation of NOX involves membrane trafficking as well as other proteins such as membrane trafficking Rops.9 A recent paper10 suggests that enhanced Rop activity can heighten ROS production regulating root hair development in Arabidopsis, as well. On the other side of our model are factors that are upregulated by GB to counteract ROS accumulation such as ROS scavenging enzymes and the processes involving RabA4c and the bZIP transcription factor. One possibility is that GB signaling upregulates membrane trafficking associated with RabA4c and that this process competes with Rop-mediated membrane trafficking associated with NOX activation.
In summary, our work suggests an alternative explanation for chilling sensitivity, emphasizing ROS signaling instead of the classical membrane phase transition model. With regard to ROS itself, the model can be seen as part of the paradigm shift during the last few years11 away from ROS as toxic molecules causing damage to proteins and DNA to ROS as signaling substances for guard cell functioning,12 photoprotection,13 pathogenesis14 and development.15
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4461
References
- 1.Lyons JM, Raison JK. Oxidative activity of mitochondria isolated from plant tissues sensitive and resistant to chilling injury. Plant Physiol. 1970;45:386–389. doi: 10.1104/pp.45.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishida I, Murata N. Chilling sensitivity in plants and cyanobacteria: The crucial contribution of membrane lipids. Annu Rev Plant Physiol. 1996;47:541–568. doi: 10.1146/annurev.arplant.47.1.541. [DOI] [PubMed] [Google Scholar]
- 3.Wu JR, Browse J. Elevated levels of high-melting-point phosphatidylglycerols do not induce chilling sensitivity in an Arabidopsis mutant. Plant Cell. 1995;7:17–27. doi: 10.1105/tpc.7.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park EJ, Jeknic Z, Sakamoto A, DeNoma J, Yuwansiri R, Murata N, Chen THH. Genetic engineering of glycinebetaine synthesis in tomato protects seeds, plants, and flowers from chilling damage. Plant J. 2004;40:474–487. doi: 10.1111/j.1365-313X.2004.02237.x. [DOI] [PubMed] [Google Scholar]
- 5.Einset J. An extracellular mechanism of light protection in plants identified using a chemical genetic screen. Acta Hort. 2006;711:339–344. [Google Scholar]
- 6.Einset J, Nielsen E, Connolly EL, Bones A, Sparstad T, Winge P, Zhu JK. Membrane trafficking RabA4c involved in the effect of glycine betaine on recovery from chilling stress in Arabidopsis. Physiol Plant. 2007 (In press) [Google Scholar]
- 7.Preuss ML, Serna J, Falbel TG, Bednarek SY, Nielsen E. The Arabidopsis Rab GTPase RabA4b localizes to the tips of growing root hair cells. Plant Cell. 2004;16:1589–1603. doi: 10.1105/tpc.021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki N, Mittler R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol Plant. 2006;126:45–51. [Google Scholar]
- 9.Bedar K, Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 10.Jones MA, Raymond MJ, Yang Z, Smirnoff N. NADPH oxidase-dependent reactive oxygen species formation required for root hair growth depends on ROP GTPase. J Exp Bot. 2007;58:1261–1270. doi: 10.1093/jxb/erl279. [DOI] [PubMed] [Google Scholar]
- 11.Apel K, Hirt H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 12.Kwak JM, Nguyen V, Schroeder JI. The role of reactive oxygen species in hormonal responses. Plant Physiol. 2006;141:323–329. doi: 10.1104/pp.106.079004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner D, Przybyla D, Camp ROD, Kim C, Landgraf F, Lee KP, Würsch M, Laloi C, Nater M, Hideg E, Apel K. The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science. 2004;306:1183–1185. doi: 10.1126/science.1103178. [DOI] [PubMed] [Google Scholar]
- 14.Torres MA, Dangl JL. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol. 2005;8:397–403. doi: 10.1016/j.pbi.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Gapper C, Dolan L. Control of plant development by reactive oxygen species. Plant Physiol. 2006;141:341–343. doi: 10.1104/pp.106.079079. [DOI] [PMC free article] [PubMed] [Google Scholar]


