Abstract
Light signals perceived by the phytochrome family of red (R) and far-red (FR) light-absorbing photoreceptors direct plant growth and development throughout their lifecycle. In contrast to other family members, phyA displays rapid light-induced proteolytic degradation upon conversion to the biologically active Pfr form and mediates high irradiance responses to continuous FR. These unique properties together with limited examples of phyA function in R have resulted in an over-simplified portrayal of phyA as a FR sensor which acts predominantly in seed germination and early stages of seedling de-etiolation. In a recent work, published in The Plant Journal, we report significant phyA activity in Arabidopsis thaliana at high (>100 µmolm−2s−1) photon irradiances of R. Under these conditions, we observed retarded degradation of a pool of nuclear-localised phyA, consistent with the phenomenon of photoprotection, and showed phyBphyCphyDphyE quadruple null mutants, containing only functional phyA, to de-etiolate and survive to flowering. The photon irradiances used in this study were greater than those routinely used for photomorphogenic analysis in the laboratory but considerably lower than those commonly observed in daylight. In this addendum we present additional analyses of the phyBphyCphyDphyE mutant and discuss the possibility that phyA may perform a significant role in the growth and development of daylight-grown plants.
Key Words: phytochrome A, red light, irradiance, hypocotyl, cotyledon, photoprotection
Introduction
Analyses of mutants, deficient in individual and multiple combinations of phytochromes, have been paramount in elucidating the functions of family members throughout plant development.1 Phytochrome A is the most abundant phytochrome in etiolated seedlings and performs a fundamental role in seedling de-etiolation.2 The rapid decrease in phyA levels upon transfer to light results from both light-mediated turnover of the protein and down-regulation of PHYA transcription.3,4 A variety of phyA-mediated very low fluence responses (VLFRs) and high irradiances responses (HIRs) to continuous far-red light (FRc) have been characterised but limited examples of phyA functions in R exist in the literature. Modestly increased hypocotyl length, reduced hook opening and reduced cotyledon expansion have all been reported in Rc-grown phyAphyB mutants when compared with monogenic phyB mutants, suggesting a role for phyA in Rc-mediated de-etiolation.5–7 In addition, phyA has been reported to mediate the R-enhancement of phototropic curvature in blue light8 and R-induced positive phototropism in roots.9 Recent microarray studies have also shown phyA to be the principle phytochrome regulating rapidly responding genes during early stages of R-mediated de-etiolation.10 The photon irradiance used in all these studies was, however <50 µmolm−2s−1. We observed considerable phyA activity at high (>100 µmolm−2s−1) photon irradiances of R. At these photon irradiances we also observed retarded degradation of the protein in wild-type (WT) plants and prolonged epifluorescence of nuclear-localised phyA::YFP in transgenic lines.
Mutants Containing only Functional phyA Display Considerable De-Etiolation at High Photon Irradiances of R
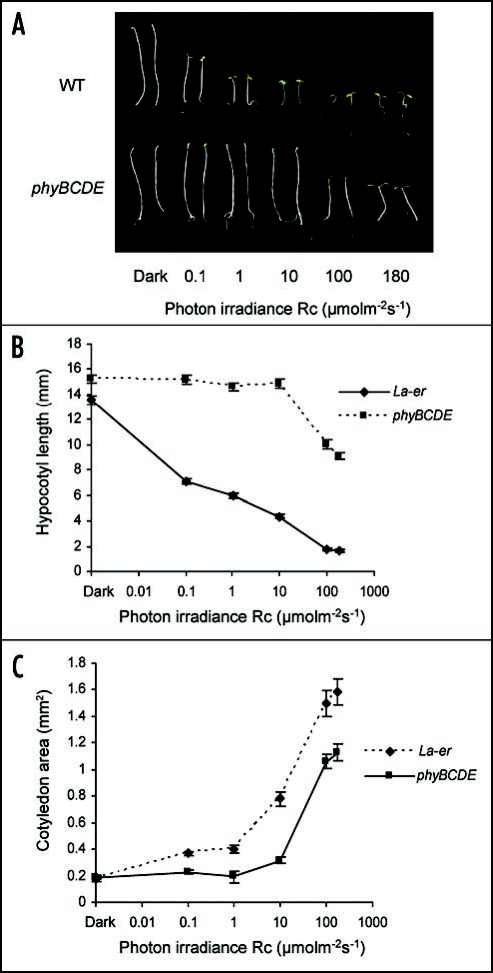
Creation of a phyBphyCphyDphyE quadruple null mutant enabled the role of phyA in R signalling to be examined in the absence of other phytochromes. The de-etiolation of these plants at a range of different photon irradiances is shown in Figure 1A. At photon irradiances >100 µmolm−2s−1 phyBphyCphyDphyE mutants displayed significant inhibition of hypocotyl elongation (Fig. 1B) and promotion of cotledon expansion (Fig. 1C). Such observations unequivocally demostrate the capacity of phyA to promote substantial de-etiolation in Rc in an irradiance-dependent manner.
Figure 1.
(A) Phenotypes of WT and phyBphyCphyDphyE seedlings grown for five days at different photon irradiances of R. (B) Hypocotyl lengths and (C) Cotyledon areas of WT and phyBphyCphyDphyE mutants grown for 5 days at different photon irradiances of R. Experimental procedures are described in Franklin et al. 2007.
A Significant Role for phyA in Daylight-Grown Plants?
Phytochrome A activity has been reported throughout the lifecycle of plants. Despite resembling WT plants in continuous white light, phyA mutants displayed elongated hypocotyls when grown in light/dark cycles and continuous low R:FR ratio.11,12 The phyA-mediated inhibition of hypocotyl growth in low R:FR ratio occurs at dawn,13 when phyA levels are highest, and is thought to ‘antagonise’ shade avoidance by preventing excessive elongation growth. This response has been shown to be of fundamental importance to seedlings developing under dense, natural vegetational shade. When grown in the field under these conditions, phyA mutants displayed extreme hypocotyl elongation, with many failing to establish and dying prematurely.14 Phytochrome A has also been shown to perform a role in the perception of daylength,2 suppression of internode elongation15 and leaf elongation16 in mature plants. This study not only demonstrates photoprotection of phyA at high photon irradiances of R (a waveband that maximises Pfr concentration and hence degradation rate) but also provides evidence of significant functional activity for photoprotected phyA. In natural daylight, photoprotection ensures maintenance of a phyA pool, despite the establishment of a relatively high Pfr concentration, conditions which maximise phyA degradation at more modest photon irradiances.17,18 Given that photoprotected phyA displays significant biological activity, it is likely that this phytochrome performs a more significant role in development of daylight-grown plants than has previously been considered.
Phytochrome-deficient mutants have proved invaluable in elucidating the functional capacity and redundant interactions of individual family members throughout development. Our understanding of how individual phytochromes functionally interact in WT plants does, however, appear partly dependent on the experimental growth conditions used for analyses. Recently, a small decrease in ambient growth temperature was shown to significantly alter the functional relationships between phytochromes for multiple physiological responses.19,20 We have now demonstrated considerable phyA activity in R, using photon irradiances higher than those commonly used for laboratory studies. Given the diverse and fluctuating environmental conditions experienced by plants growing in natural communities, it is likely that further broadening of experimental regimes may reveal additional functional activities for individual phytochromes, thus providing additional insight in to the regulatory roles of this important group of plant photoreceptors.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4261
References
- 1.Franklin KA, Larner VS, Whitelam GC. The signal transducing photoreceptors of plants. Int J Dev Biol. 2005;49:653–664. doi: 10.1387/ijdb.051989kf. [DOI] [PubMed] [Google Scholar]
- 2.Smith H, Whitelam GC. Phytochrome, a family of photoreceptors with multiple physiological roles. Plant Cell Environ. 1990;13:695–707. [Google Scholar]
- 3.Clough RC, Vierstra RD. Phytochrome degradation. Plant Cell Environ. 1997;20:713–721. [Google Scholar]
- 4.CantÓn FR, Quail PH. Both phyA and phyB mediate light-imposed repression of PHYA gene expression in Arabidopsis. Plant Physiol. 1999;121:1207–1215. doi: 10.1104/pp.121.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parks BM, Quail PH, Hangarter RP. Phytochrome A regulates red-light induction of phototropic enhancement in Arabidopsis. Plant Physiol. 1996;110:155–162. doi: 10.1104/pp.110.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiss JZ, Mullen JL, Correll MJ, Hangarter RP. Phytochrome A and B mediate red-light-induced positive phototropism in roots. Plant Physiol. 2003;131:1411–1417. doi: 10.1104/pp.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casal JJ, Mazzella MA. Conditional synergism between cryptochrome 1 and phytochrome B is shown by the analysis of phyA, phyB and hy4 simple, double and triple mutants in Arabidopsis. Plant Physiol. 1998;118:19–25. doi: 10.1104/pp.118.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neff MM, Chory J. Genetic interaction between phytochrome A, phytochrome B and cryptochrome 1 during Arabidopsis development. Plant Physiol. 1998;118:27–36. doi: 10.1104/pp.118.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell. 1994;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tepperman JM, Hwang Y-S, Quail PH. phyA dominates in transduction of red-light signals to rapidly responding genes at the initiation of Arabidopsis seedling de-etiolation. Plant J. 2006;48:728–742. doi: 10.1111/j.1365-313X.2006.02914.x. [DOI] [PubMed] [Google Scholar]
- 11.Whitelam GC, Johnson E, Peng J, Carol P, Anderson ML, Cowl JS, Harberd NP. Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell. 1993;5:757–768. doi: 10.1105/tpc.5.7.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson E, Bradley M, Harberd NP, Whitelam GC. Photoresponses of light-grown phyA mutants of Arabidopsis. Plant Physiol. 1994;105:141–149. doi: 10.1104/pp.105.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salter MG, Franklin KA, Whitelam GC. Gating of the rapid shade avoidance response by the circadian clock in plants. Nature. 2003;426:680–683. doi: 10.1038/nature02174. [DOI] [PubMed] [Google Scholar]
- 14.Yanovsky MJ, Casal JJ, Whitelam GC. Phytochrome A, phytochrome B and HY4 are involved in hypocotyl growth responses to natural radiation in Arabidopsis: weak de-etiolation of the phyA mutant under dense canopies. Plant Cell Environ. 1995;18:788–794. [Google Scholar]
- 15.Devlin PF, Patel SR, Whitelam GC. Phytochrome E influences internode elongation and flowering time in Arabidopsis. Plant Cell. 1998;10:1479–1487. doi: 10.1105/tpc.10.9.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franklin KA, Praekelt U, Stoddart WM, Billingham OE, Halliday KJ, Whitelam GC. Phytochromes B, D and E act redundantly to control multiple physiological responses in Arabidopsis. Plant Physiol. 2003;131:1340–1346. doi: 10.1104/pp.102.015487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kendrick RE, Spruit CJP. Phytochrome decay in seedlings under continuous incandescent light. Planta. 1972;107:341–350. doi: 10.1007/BF00386395. [DOI] [PubMed] [Google Scholar]
- 18.Smith H, Jackson M, Whitelam GC. Photoprotection of phytochrome. Planta. 1988;175:471–477. doi: 10.1007/BF00393067. [DOI] [PubMed] [Google Scholar]
- 19.Halliday KJ, Salter MG, Thingnaes E, Whitelam GC. Phytochrome control of flowering is temperature sensitive and correlates with expression of the floral integrator FT. Plant J. 2003;33:875–885. doi: 10.1046/j.1365-313x.2003.01674.x. [DOI] [PubMed] [Google Scholar]
- 20.Halliday KJ, Whitelam GC. Changes in photoperiod or temperature reveal roles for phyD and phyE. Plant Physiol. 2003;131:1913–1920. doi: 10.1104/pp.102.018135. [DOI] [PMC free article] [PubMed] [Google Scholar]