Abstract
A high-throughput in planta overexpression screen of a Nicotiana benthamiana cDNA library identified a mitogen activated protein kinase kinase (MAPKK), NbMKK1, as a potent inducer of hypersensitive response (HR)-like cell death. NbMKK1-mediated cell death was attenuated in plants whereby expression of NbSIPK, an ortholog of tobacco SIPK and Arabidopsis AtMPK6, was knocked down by virus-induced gene silencing (VIGS), suggesting that NbMKK1 functions upstream of NbSIPK. In accordance with this result, NbMKK1 phosphorylated NbSIPK in vitro, and furthermore NbMKK1 and NbSIPK physically interacted in yeast two-hybrid assay. VIGS of NbMKK1 in N. benthamiana resulted in a delay of Phytophthora infestans INF1 elicitin-mediated HR as well as in the reduction of resistance against a non-host pathogen Pseudomonas cichorii. Our data of NbMKK1, together with that of LeMKK4,1 demonstrate the presence of a novel defense signaling pathway involving NbMKK1/LeMKK4 and SIPK.
Key Words: MAPK, defense, cell death, in planta screen
Mitogen activated protein kinase (MAPK) cascades are highly conserved signaling pathways in eukaryotes, comprising three tiered classes of protein kinase, MAPKKK (MAPKK kinase), MAPKK and MAPK, that sequentially relay phosphorylation signals.2 The Arabidopsis genome carries genes for 20 MAPKs, 10 MAPKKs3 and more than 25 MAPKKKs.4 In plants, MAPK signaling is known to function in various biotic4,5 and abiotic6 stress responses and cytokinesis.7 In defense signaling, extensive research has been carried out for two tobacco MAPKs, SIPK8 (salicylic-acid-induced protein kinase; hereafter designated as NtSIPK) and WIPK9 (wound-induced protein kinase = NtWIPK), and their orthologs in Arabidopsis10 (AtMPK6 and ATMPK3, respectively), partly because kinase activities of these two MAPKs are easy to detect by an in gel kinase assay using myeline basic protein (MBP) as substrate.11 Both NtSIPK and NtWIPK are activated by the interaction between host resistance (R)- gene and cognate avirulence gene of pathogen11,12 and elicitor perception by host cells.13,14 Shuqun Zhang and his group showed that an upstream kinase of both NtSIPK and NtWIPK is NtMEK2.15 Transient overexpression of constitutively active NtMEK2 caused phosphorylation of NtSIPK and NtWIPK, resulting in rapid HR-like cell death in tobacco leaves.15 Later, the same lab showed that overexpression of NtSIPK alone also caused HR-like cell death.16 The downstream target proteins of NtSIPK and AtMPK6 are being identified and include 1-aminocyclopropane-1-carboxylic acid sythase-6 (ACS-6).17,18 Although recent studies identified another MAPK cascade (NtMEK1 → Ntf6) involved in defense responses19,20 we can still say that the current research focus of MAPK defense signaling centers around the cascade comprising [NtMEK2→ NtSIPK/NtWIPK→ target proteins] of tobacco and its orthologous pathways in other plant species.
In an effort to search for plant genes involved in HR-like cell death, we have been employing a high-throughput in planta expression screen of N. benthamiana cDNA libraries. In this experimental system, a cDNA library was made in a binary potato virus X (PVX)-based expression vector pSfinx.21 The cDNA library was transferred to Agrobacterium tumefaciens, and 40,000 of the bacterial colonies were individually inoculated by toothpicks onto leaf blades of N. benthamiana leaves. The phenotype around the inoculated site was observed 1–2 weeks following the inoculation. This rapid screen identified 30 cDNAs that caused cell death after overexpression, including genes coding for ubiquitin proteins, RNA recognition motif (RRM) containing proteins, a class II ethylene-responsive element binding factor (EREBP)-like protein22 and a MAPKK protein (this work). Such an in planta screening technique has been used before for the isolation of fungal21 and oomycete23,24 elicitors and necrosis inducing genes, but not for isolation of plant genes. Overexpression screening of cDNA libraries is a common practice in prokaryotes, yeast and amimal cells,25,26 so it is a surprise that this approach has not been systematically applied in plants. Given its throughput, we propose that this virus-based transient overexpression system is a highly efficient way to isolate novel plant genes by functional screen.27 Since overexpression frequently causes non-specific perturbation of signaling, genes identified by overexpression should be further validated by loss-of-function assays, for instance, VIGS.28
Overexpression of the identified MAPKK gene, NbMKK1, triggered a rapid generation of H2O2, followed by HR-like cell death in N. benthamiana leaves (this work). NbMKK1-GFP fusion protein overexpression also caused cell death, and curiously NbMKK1-GFP was shown to localize consistently in the nucleus. Sequence comparison classified NbMKK1 to the Group D of MAPKKs about which little information is available. So far, a MAPKK, LeMKK4, from tomato belonging to the Group D MAPKKs, was shown to cause cell death after overexpression.1 Based on amino acid sequence similarity and phylogenetic analyses, LeMKK4 and NbMKK1 seem to be orthologs. To see whether NbMKK1 transduces signals through SIPK and WIPK, we performed NbMKK1 overexpression in N. benthamiana plants whereby the expression of either NbSIPK or NbWIPK (WIPK ortholog in N. benthamiana) was silenced by VIGS. NbMKK1 did not induce cell death in NbSIPK-silenced plants, suggesting that the NbMKK1 cell death signal is transmitted through NbSIPK. Indeed, NbMKK1 phosphorylated NbSIPK in vitro, and NbMKK1 and NbSIPK physically interacted in yeast two-hybrid assay. These results suggest that NbMKK1 interacts with NbSIPK, most probably with its N-terminal docking domain, and phosphorylates NbSIPK in vivo to transduce the cell death signal downstream.
NbMKK1 exhibits constitutive expression in leaves. To determine the function of NbMKK1 in defense, we silenced NbMKK1 by VIGS, and such plants were challenged with Phytophthora infestans INF1 elicitin29 and Pseudomonas cichorii, a non-host pathogen. INF1-mediated HR cell death was remarkably delayed in NbMKK1-silenced plants. Likewise, plant defense against P. cichorii was compromised in NbMKK1-silenced plants. These results indicate that NbMKK1 is an important component of signaling of INF1-mediated HR and non-host resistance to P. cichorii.
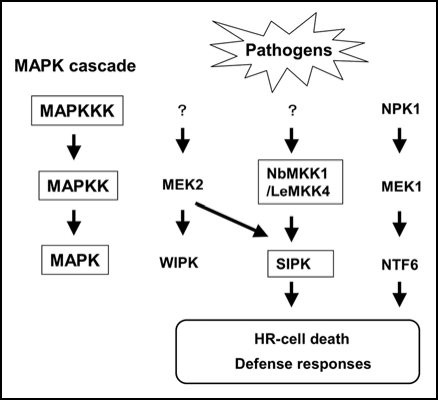
Together, our analyses of NbMKK1 and independent work from Greg Martin's lab on LeMKK41 suggest that a Group D MAPKK, NbMKK1/LeMKK4, functions upstream of SIPK and transduces defense signals in these solanaceous plants (Fig. 1). In plants as well as in other eukaryotes, it is common that kinases have multiple partners. The work on these kinases fits this concept. A single MAPK (e.g., SIPK) is phosphorylated by multiple MAPKKs (e.g., NtMEK2 and NbMKK1), and a single MAPKK (e.g., NtMEK2) can phosphorylate multiple MAPKs (e.g., NtSIPK and NtWIPK).
Figure 1.
Defense signaling through NbMKK1/LeMKK4. Two defense signal pathways involving NtMEK2 (indicated as MEK2) → WIPK/SIPK and NtMEK1(indicated as MEK1) → Ntf6 are well documented. By our and Pedley and Martin's1 works, another novel MAPKK, NbMKK1/LeMKK4 was demonstrated to participate in defense signaling by phosphorylation of SIPK.
Acknowledgements
We acknowledge ‘Program for Promotion of Basic Research Activities for Innovative Bioscience’ for supporting the research and Matt Shenton for improving the manuscript.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4267
References
- 1.Pedley KF, Martin GF. Identification of MAPKs and their possible MAPK kinase activators involved in the Pto-mediated defense response of tomato. J Biol Chem. 2004;279:49229–49235. doi: 10.1074/jbc.M410323200. [DOI] [PubMed] [Google Scholar]
- 2.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: Conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 3.MAPK Group, author. Mitogen-activated protein kinase cascades in plants: A new nomenclature. Trends Plant Sci. 2002;7:301–308. doi: 10.1016/s1360-1385(02)02302-6. [DOI] [PubMed] [Google Scholar]
- 4.Tena G, Asai T, Chiu WL, Sheen J. Plant mitogen-activated protein kinase signaling cascades. Curr Opin Plant Biol. 2001;4:392–400. doi: 10.1016/s1369-5266(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 5.Pedley KF, Martin GB. Role of mitogen-activated protein kinases in plant immunity. Curr Opin Plant Biol. 2005;8:541–547. doi: 10.1016/j.pbi.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Nakagami H, Pitzschke A, Hirt H. Emerging MAP kinase pathways in plant stress signalling. Trends Plant Sci. 2005;10:339–346. doi: 10.1016/j.tplants.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Nishihama R, Machida Y. Expansion of the phragmoplast during plant cytokinesis: A MAPK pathway may MAP it out. Current Opinion in Plant Biology. 2001;4:507–512. doi: 10.1016/s1369-5266(00)00208-9. [DOI] [PubMed] [Google Scholar]
- 8.Zhang S, Klessig DF. Salicylic acid activates a 48 kD MAP kinase in tobacco. Plant Cell. 1997;9:809–824. doi: 10.1105/tpc.9.5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seo S, Okamoto M, Seto H, Ishizuka K, Sano H, Ohashi Y. Tobacco MAP kinase: A possible mediator in wound signal transduction pathways. Science. 1995;270:1988–1992. doi: 10.1126/science.270.5244.1988. [DOI] [PubMed] [Google Scholar]
- 10.Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. MAP kinase signalling cascade in Arabisopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- 11.Romeis T, Piedras P, Zhang S, Klessig DF, Hirt H, Jones JDG. Rapid Avr9- and Cf9-dependent activation of MAP kinases in tobacco cell cultures and leaves: Convergence of resistance gene, elicitor, wound, and salicylate responses. Plant Cell. 1999;11:273–287. doi: 10.1105/tpc.11.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S, Klessig DF. N resistance gene-mediated de novo synthesis and activation of a tobacco MAP kinase by TMV infection. Proc Natl Acad Sci USA. 1998;95:7433–7438. doi: 10.1073/pnas.95.13.7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J, Klessig DF, Nurnberger T. A harpin binding site in tobacco plasma membranes mediates activation of the pathogenesis-related gene HIN1 independent of extracellular calcium but dependent on mitogen-activated protein kinase activity. Plant Cell. 2001;13:1079–1093. doi: 10.1105/tpc.13.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S, Liu Y, Klessig DF. Multiple levels of tobacco WIPK activation during the induction of cell death by fungal elicitins. Plant J. 2000;23:339–347. doi: 10.1046/j.1365-313x.2000.00780.x. [DOI] [PubMed] [Google Scholar]
- 15.Yang KY, Liu Y, Zhang S. Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc Natl Acad Sci USA. 2001;98:741–746. doi: 10.1073/pnas.98.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S, Liu Y. Activation of salicylic acid-induced protein kinase, a mitogen-activated protein kinase, induces multiple defense responses in tobacco. Plant Cell. 2001;13:1877–1889. doi: 10.1105/TPC.010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Zhang S. Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell. 2004;16:3386–3399. doi: 10.1105/tpc.104.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feilner T, Hultschig C, Lee J, Meyer S, Immink RGH, Koenig A, Possling A, Seitz H, Beveridge A, Scheel D, Cahill DJ, Lehrach H, Kreutzberger J, Kersten B. High throughput identification of potential Arabidopsis mitogen-activated protein kinase substrates. Mol Cell Proteomics. 2005;4:1558–1568. doi: 10.1074/mcp.M500007-MCP200. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Shiff M, Dinesh-Kumar SP. Involvement of MEK1 MAPKK, NTF6 MAPK, WRKY/MYB transcription factors, COI1 and CTR1 in N-mediated resistance to tobacco mosaic virus. Plant J. 2004;38:800–809. doi: 10.1111/j.1365-313X.2004.02085.x. [DOI] [PubMed] [Google Scholar]
- 20.Ekengren SK, Liu Y, Schiff M, Dinesh-Kumar SP, Martin GB. Two MAPK cascades, NPR1, and TGA transcription factors play a role in Pto-mediated disease resistance in tomato. Plant J. 2003;36:905–917. doi: 10.1046/j.1365-313x.2003.01944.x. [DOI] [PubMed] [Google Scholar]
- 21.Takken FLW, Luderer R, Gabriels SHEJ, Westerink N, Lu R, de Wit PJGM, Joosten MHAJ. A functional cloning strategy, based on a binary PVX-expression vector, to isolate HR-inducing cDNAs of plant pathogens. Plant J. 2000;24:275–283. doi: 10.1046/j.1365-313x.2000.00866.x. [DOI] [PubMed] [Google Scholar]
- 22.Nasir KHB, Takahashi Y, Ito A, Saitoh H, Matsumura H, Kanzaki H, Shimizu T, Ito M, Fujisawa S, Sharma PC, Ohme-Takagi M, Kamoun S, Terauchi R. High-throughput in planta expression screening identifies a class II ethylene-responsive element binding factor-like protein that regulates plant cell death and non-host resistance. Plant J. 2005;43:491–505. doi: 10.1111/j.1365-313X.2005.02472.x. [DOI] [PubMed] [Google Scholar]
- 23.Torto TA, Li S, Styer A, Huitema E, Testa A, Gow NAR, van West P, Kamoun S. EST mining and functional expression assays identify extracellular effector proteins from the plant pathogen Phytophthora. Genome Res. 2003;13:1675–1685. doi: 10.1101/gr.910003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huitema E, Bos JIB, Tian M, Win J, Waugh ME, Kamoun S. Linking sequence to phenotype in Phytophthora-plant interactions. Trends Microbiol. 2004;12:193–200. doi: 10.1016/j.tim.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Grimm S. The art and design of genetic screens: Mammalian culture cells. Nat Rev Genet. 2004;5:179–189. doi: 10.1038/nrg1291. [DOI] [PubMed] [Google Scholar]
- 26.Rine J. Gene overexpression in studies of Saccharomyces cerevisiae. Methods Enzymol. 1991;194:239–251. doi: 10.1016/0076-6879(91)94019-9. [DOI] [PubMed] [Google Scholar]
- 27.Terauchi R, Bin Nasir KH, Ito A, Saitoh H, Berberich T, Takahashi Y. High-throughput functional screening of plant and pathogen genes in planta. Plant Biotech. 2005;22:455–459. [Google Scholar]
- 28.Baulcombe DC. Fast forward genetics based on virus-induced gene silencing. Curr Opin Plant Biol. 1999;2:109–113. doi: 10.1016/S1369-5266(99)80022-3. [DOI] [PubMed] [Google Scholar]
- 29.Kamoun S, van West P, Vleeshouwers VG, de Groot KE, Govers F. Resistance of Nicotiana benthamiana to Phytophthora infestans is mediated by the recognition of the elicitor protein INF1. Plant Cell. 1998;10:1413–1426. doi: 10.1105/tpc.10.9.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]