Abstract
Plasmodesmata (Pd), coaxial membranous channels that connect adjacent plant cells, are not static, but show a dynamic nature and can be opened or closed. These controlled changes in Pd conductivity regulate plant symplasmic permeability and play a role both in development and defense processes. One of the mechanisms shown to produce these changes is the deposition and hydrolysis of callose by β-1-3-synthase and glucanase, respectively. Recently we have identified the first β-1,3-glucanase Arabidopsis enzyme that is associated to the macromolecular Pd complex, termed AtBG_pap. When fused to GFP, this previously identified GPI-anchored protein localizes to the ER and the plasma membrane where it appears in a punctuate pattern that colocalizes with callose present around Pd. In T-DNA insertion mutants that do not transcribe AtBG_pap, GFP cell-to-cell movement between epidermal cells is reduced and callose levels around Pd are elevated. In this addenda we review the plant developmental processes of symplasmic regulation that have been shown to include callose deposition and β-1,3-glucanase activity, and suggest a role for AtBG_pap in these processes. Additionally, based on the ability of viral movement proteins (MPs) to interact with ankyrin repeat proteins, and together with our recent findings showing the involvement of viral particles in callose degradation, we also purpose a new model for the ability of viruses to overcome Pd-callose deposition, and mediate their cell-to-cell movement.
Key Words: plasmodesmata; cell-cell communication; callose; β-1,3-glucanase; movement protein; ankyrin repeats
Callose Turnover as a Regulator of Pd
Plasmodesmata (Pd), plasma-membrane-lined channels that connect plant cells, are not static organelles, but rather show a high degree of plasticity and can change in a transient manner from having ‘closed’ to ‘open’ to ‘dilated’ state. The dynamic properties of Pd play an important role in regulating the direct cell-to-cell transport of molecules between cells, in providing a cell-to-cell passageway for plant viruses and in the organization and functioning of symplasmic domains. One of the mechanisms proposed to produce these focused changes in the channels is the deposition and hydrolysis of the β-1,3-glucan poly-sugar molecule callose found in the cell-wall sheath surrounding Pd orifices.1,2 Reversible deposition of callose in the Pd is found both during developmental processes3,4 and in response to stress.5,6 Several studies have shown that accumulation of callose around Pd results in decreased cell-to-cell movement of fluorescent dyes,5,7,8 while treatments inhibiting callose deposition resulted in a doubling in the diameter of Pd orifices and a higher Pd size exclusion limit (SEL, the size of the largest molecule that will move cell-to-cell).6,7 Additional support for this proposed model comes from works employing β-1,3-glucanases, enzymes that degrade callose. In studies with a tobacco mutant that had decreased levels of class I β-1,3-glucanase, generated by antisense transformation, and which exhibited increased levels of callose, monitoring the cell-to-cell movement of dextrans and peptides showed that SEL of Pd was reduced.9
Recently, we published a paper10 describing the identification of the first β-1,3-glucanase enzyme that is associated with Pd from Arabidopsis. This protein, termed AtBG-pap (plasmodesmal associated protein) was found by MS/MS proteomic analysis of a Pd enriched fraction that was obtained by cellulase enzymatic digestion of isolated cell walls from Arabidopsis.
AtBG-pap, when expressed as a fusion protein to green fluorescent protein (GFP) in transgenic Nicotiana tabacum epidermal cells, localizes to the cell membrane, and is highly enriched at Pd sites, presumably in the plasma-membrane component. In spongy mesophyll cells the punctuate fluorescence is seen only in walls that connect adjacent cells. AtBG-pap is a glycosylphosphatidylinositol (GPI) lipid-anchored protein, and thus is expected to be attached to the outer leaflet of the plasma membrane. This localization places the enzyme facing the cell wall sheath around the Pd (which contains callose), enabling it to degrade callose in the extracellular wall region adjacent to the cellular membrane.
If callose hydrolysis indeed increases the aperture of the Pd channels, then abolishing the expression of β-1,3-glucanase should result in a steady state increase in callose accumulation around Pd reducing the free space available for passage. To test this influence, we measured the diffusive movement of free GFP expressed in a single cell to surrounding epidermal leaf cells of AtBG-pap T-DNA mutant Arabidopsis plants. Both the number of cells to which GFP diffused and the coefficient of conductivity of Pd were lower in the AtBG-pap mutants. In addition, the AtBG-pap mutants accumulate higher levels of callose around Pd two days after wounding.
These results provide strong support to the supposition that callose turnover regulates Pd SEL, and that this turnover is the result of synthesis vs. hydrolysis activities. The identity of the Pd specific callose synthase is still unresolved.
β-1,3-Glucanases, Symplasmic Regulation and Development
The Arabidopsis β-1,3-glucanase family is comprised of 50 individual genes. A recent work grouped 44 genes from this large family into 13 expression clusters denoted A–M based on microarray data.11 Groups A–D contain β-1,3-glucanases specific to leaves (A–C) and roots (D). Proteins of these groups displayed the most significant responses to fungal pathogens, and are most likely to contain pathogenic related (PR)-genes whose transcription is upregulated following pathogen infection. Groups H and K include genes with expression pattern specific to flower organs, and which are likely to be involved in reproductive processes such as microspore maturation and pollen tube growth. AtBG-pap belongs to the largest identified group (M), which contains 13 genes whose expression products are abundant in a variety of tissues with a high relative expression in the shoot-apex. Members of this group are thus suggested to be involved in cell wall morphogenesis or cell division. Group M members display only a minimal response to most stresses and hormones, and a slight negative response to biotic stresses, and thus are not likely to have a PR role. AtBG-pap RNA measurements following infection with either Cucumber mosaic virus (CMV) or Pseudomonas syringae pv. tomato showed no induction or repression of transcription of AtBG-pap gene (unpublished data), also excluding a PR role. The Pd location of AtBG-pap thus suggests a role in constitutive regulation of Pd conductivity.
Several processes of symplasmic communication regulation have been shown to include callose deposition and β-1,3-glucanase activity. For example, during dormancy development in birch, a perennial plant, which is activated by exposure to short days, the Pd of the shoot apical meristem are firmly closed by intra- and extra-cellular structures that contain callose.4,12 After adequate chilling Pd regain their capacity to transport fluorescent dyes between cells, and callose containing sphincters disappear. During this process β-1,3-glucanase proteins were upregulated, and were localized in spherosome-like vacuoles or lipid bodies in the vicinity of the cell membrane and in contact with Pd.12 Similar events take place in the phloem of perennials in which the sieve-plate pores become plugged in autumn by the deposition of β-1,3- glucan, and reopen in spring by the activation of β-1,3-glucanase.13
Another process that involves induction of β-1,3-glucanase is germination. In germinating tobacco seeds β-1,3-glucanase protein and RNA levels are induced just prior to endosperm rupture. This induction is highly localized in the micropylar endosperm at the site of radicle emergence, and is effected by physiological factors known to affect the incidence and timing of germination (light, gibberellins, ethylene, abscisic acid).14 While the specific activity of β-1,3-glucanase in germination is still unknown, it was proposed that β-1,3-glucanase may contribute to the release of seed dormancy by the hydrolysis of Pd callose, thus activating the symplasmic connections between cells.
In all the examples above the involvement of β-1,3-glucanase in symplasmic regulation has been described mainly in processes that are more persistent and permanent. This may indicate that the plant uses the callose turnover mechanism for longer-lasting and stable closure of Pd, and may utilize other mechanisms for faster changes in the channels.
β-1,3-Glucanases and Viral Cell-to-Cell Spread
Many viruses move cell-to-cell with the help of one or more viral encoded movement proteins (MPs) that target to and dilate Pd.15 It was proposed that this activity enables movement of the virus through the channel. However, the mechanism by which MP increases Pd SEL is still unknown.
Measurements of callose levels around Pd, revealed that infection of N. tabacum with a minimal replicon of tobacco mosaic virus lacking coat protein and MP (TMVΔCPΔMP) led to the deposition of callose around the channels. While expression of the TMV MP alone had no effect on callose levels and Pd opening in MP transgenic N. tabacum, infection of the MP transgenic plants with the TMVΔCPΔMP replicon led to reduced callose accumulation (unpublished data). This suggests that callose deposition arise largely as a result of a plant defense mechanism aimed at restricting virus spread, and that MP synergistically with viral replicase function in mediating the degradation of the callose, thus opening Pd. To achieve this, the virus must somehow recruit and/or activate β-1,3-glucanase at the Pd.
The importance of β-1,3-glucanase in the cell-to-cell movement of viruses was shown in studies with a tobacco mutant that had decreased levels of a class I β-1,3-glucanase generated by antisense transformation. In this mutant line, susceptibility to virus infection was decreased.9 Moreover, when the β-1,3-glucanase coding sequence was cloned into TMV, the virus spread faster through the cells, and cloning of the gene in an antisense formation led to the opposite results.16 However, class I β-1,3-glucanase enzymes are targeted to the endoplasmic reticulum (ER) lumen and the vacuole, so their potential activity at Pd requires the presence of a still unknown targeting mechanism.
In a yeast two-hybrid screen to identify proteins that bind to the potato virus x (PVX) triple-block protein TGB12K required for cell-to-cell movement, three tobacco host factors termed TBG 12K interacting proteins (TIPs) were identified.17 All three TIPs were also shown to interact with the class I vacuolar β-1,3-glucanase. In Nicotiana plumbaginifolia a yeast two hybrid screen had identified two Glucanohydrolase Binding Proteins (GBPs) that bind class I β-1,3-glucanase and chitinase.18 All five identified proteins belong to the family of ‘ankyrin-repeats’ (AKR), which contains domains that serve for protein-protein interactions. From these results it seemed likely that AKR could serve as a link between MPs and β-1-3-glucanases. It can be purposed that by binding of AKR with both MP and β-1,3-glucanase, viral MP is able to direct class I β-1,3-glucanase to Pd. However, the ability of AKR to bind β-1,3-glucanase may only occur in vitro. While AKRs identified are cytoplasmic proteins,17,18 class I β-1,3-glucanase are vacuolar or ER luminal proteins prior to being trafficked to the vacuole.12,19 As AKRs and class I β-1,3-glucanases localize at different cellular compartments, an in vivo interaction between them seems, at present, unlikely.18
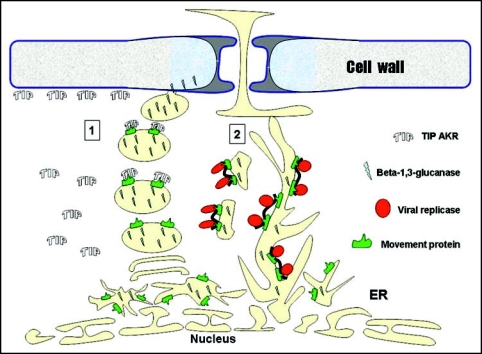
So can the virus use AKRs to target class I β-1,3-glucanases to the Pd? Unlike β-1,-3-glucanase, viral MPs are membranal proteins localized in the ER membrane system.15 Thus, an interaction between the cytoplasmic region of MP and AKR in-vivo is possible. We suggest that the binding of PVX MP by AKR could serve as a mechanism to target ER-derived vesicles that contain class I β-1,3-glucanases to the cell wall (Fig. 1). This model fits with the findings that PVX MP induces the deposition of additional cytoplasm (that contains TIP AKRs) at the cell periphery.17 We further suggest that when MP is bound to viral RNA it is targeted to the cortical ER allowing the protein complex to move cell-to-cell associated with the Pd ER compartment.
Figure 1.
Hypothesized model for viral Pd targeting and callose hydrolysis in tobacco. Early in infection, viral RNA replicates in association with ER membranes through viral replicase. Viral replication induces a PR reaction resulting in callose accumulation around Pd. Viral RNA synthesizes MP which incorporates as a intrinsic membrane protein into ER and ER derived vesicle bodies, both of which also contain β-1,3-glucanase (e.g., ER-derived sphrosome-like bodies ref. 12). MPs can then function in two different pathways; 1. MP binds TIPs cytoplasmic proteins which are involved in directing ER-vesicles to the cell periphery (see ref. 17) adjacent to the Pd. The vesicles, containing β-1,3-glucanase cargo, fuse to plasma membrane and deliver β-1,3-glucanase to the cell wall. Callose is hydrolyzed allowing Pd to dilate. Vesicles recycle back to the ER; 2. The ER with embedded MP binds viral RNA and replicase. Vesicles are formed and transported to the cortical ER and Pd. Vesicles fuse to cortical ER. The complex of MP with viral RNA and virus replicase diffuse in ER to adjacent cell.
A deeper knowledge of the mechanisms that control β-1,3 glucanase activity and callose turnover in the Pd will enrich our understanding of all the plant processes which involve symplasmic transport and communication.
Abbreviations
- Pd
plasmodesmata
- SEL
size exclusion limit
- GFP
green fluorescent protein
- GPI
glycosylphosphatidylinositol
- PR
pathogenic related
- CMV
cucumber mosaic virus
- MP
movement protein
- TMV
tobacco mosaic virus
- ER
endoplasmic reticulum
- PVX
potato virus x
- TIP
TBG 12K interacting protein
- GBP
glucanohydrolase binding protein
- AKR
ankyrin-repeats
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4334
References
- 1.Turner A, Wells B, Roberts K. Plasmodesmata of maize root tips: Structure and composition. J Cell Sci. 1994;107:3351–3361. doi: 10.1242/jcs.107.12.3351. [DOI] [PubMed] [Google Scholar]
- 2.Heinlein M, Epel BL. Macromolecular transport and signaling through plasmodesmata. Int Rev Cytol. 2004;235:93–164. doi: 10.1016/S0074-7696(04)35003-5. [DOI] [PubMed] [Google Scholar]
- 3.Ruan YL, Xu SM, White R, Furbank RT. Genotypic and developmental evidence for the role of plasmodesmatal regulation in cotton fiber elongation mediated by callose turnover. Plant Physiol. 2004;136:4104–4113. doi: 10.1104/pp.104.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rinne PLH, van der Schoot C. Symplasmic fields in the tunica of the shoot apical meristem coordinate morphogenetic events. Development. 1998;125:1477–1485. doi: 10.1242/dev.125.8.1477. [DOI] [PubMed] [Google Scholar]
- 5.Sivaguru M, Fujiwara T, Samaj J, Baluska F, Yang ZM, Osawa H, Maeda T, Mori T, Volkmann D, Matsumoto H. Aluminum-induced 1-> 3-b-D-glucan inhibits cell-to-cell trafficking of molecules through plasmodesmata: A new mechanism of aluminum toxicity in plants. Plant Physiol. 2000;124:991–1005. doi: 10.1104/pp.124.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radford JE, Vesk M, Overall RL. Callose deposition at plasmodesmata. Protoplasma. 1998;201:30–37. [Google Scholar]
- 7.Radford JE, White RG. Effects of tissue-preparation-induced callose synthesis on estimates of plasmodesma size exclusion limits. Protoplasma. 2001;216:47–55. doi: 10.1007/BF02680130. [DOI] [PubMed] [Google Scholar]
- 8.Rinne PLH, van den Boogaard R, Mensink MGJ, Kopperud C, Kormelink R, Goldbach R, van der Schoot C. Tobacco plants respond to the constitutive expression of the tospovirus movement protein NSM with a heat-reversible sealing of plasmodesmata that impairs development. Plant J. 2005;43:688–707. doi: 10.1111/j.1365-313X.2005.02489.x. [DOI] [PubMed] [Google Scholar]
- 9.Iglesias VA, Meins F., Jr Movement of plant viruses is delayed in a beta-1,3-glucanase-deficient mutant showing a reduced plasmodesmatal size exclusion limit and enhanced callose deposition. Plant J. 2000;21:157–166. doi: 10.1046/j.1365-313x.2000.00658.x. [DOI] [PubMed] [Google Scholar]
- 10.Levy A, Erlanger M, Rosenthal M, Epel BL. A plasmodesmata-associated beta-1,3-glucanase in Arabidopsis. Plant J. 2007;49:669–682. doi: 10.1111/j.1365-313X.2006.02986.x. [DOI] [PubMed] [Google Scholar]
- 11.Doxey AC, Yaish MWF, Moffatt BA, Griffith M, McConkey BJ. Functional divergence in the Arabidopsis beta-1,3-glucanase gene family inferred by phylogenetic reconstruction of expression states. Mol Biol Evol. 2007;24:1045–1055. doi: 10.1093/molbev/msm024. [DOI] [PubMed] [Google Scholar]
- 12.Rinne PLH, Kaikuranta PM, van der Schoot C. The shoot apical meristem restores its symplasmic organization during chilling-induced release from dormancy. Plant J. 2001;26:249–264. doi: 10.1046/j.1365-313x.2001.01022.x. [DOI] [PubMed] [Google Scholar]
- 13.Krabel D, Eschrich W, Wirth S, Wolf G. Callase-(1,3-Beta-D-glucanase) activity during spring reactivation in deciduous trees. Plant Sci. 1993;93:19–23. [Google Scholar]
- 14.Leubner-Metzger G. Functions and regulation of beta-1,3-glucanases during seed germination, dormancy release and after-ripening. Seed Sci Res. 2003;13:17–34. [Google Scholar]
- 15.Beachy RN, Heinlein M. Role of P30 in replication and spread of TMV. Traffic. 2000;1:540–544. doi: 10.1034/j.1600-0854.2000.010703.x. [DOI] [PubMed] [Google Scholar]
- 16.Bucher GL, Tarina C, Heinlein M, Di Serio F, Meins F, Jr, Iglesias VA. Local expression of enzymatically active class I beta-1, 3-glucanase enhances symptoms of TMV infection in tobacco. Plant J. 2001;28:361–369. doi: 10.1046/j.1365-313x.2001.01181.x. [DOI] [PubMed] [Google Scholar]
- 17.Fridborg I, Grainger J, Page A, Coleman M, Findlay K, Angell S. TIP, a novel host factor linking callose degradation with the cell-to-cell movement of Potato virus X. Mol Plant Microbe In. 2003;16:132–140. doi: 10.1094/MPMI.2003.16.2.132. [DOI] [PubMed] [Google Scholar]
- 18.Wirdnam C, Motoyama A, Arn-Bouldoires E, van Eeden S, Iglesias A, Meins F. Altered expression of an ankyrin-repeat protein results in leaf abnormalities, necrotic lesions, and the elaboration of a systemic signal. Plant Mol Biol. 2004;56:717–730. doi: 10.1007/s11103-004-4679-9. [DOI] [PubMed] [Google Scholar]
- 19.Leubner-Metzger G, Meins F. Functions and regulation of plant beta-1,3-glucanases (PR-2) In: Datta SK, Muthukrishnan S, editors. Pathogenesis-related proteins in plants. Florida: CRC Press; 1999. pp. 49–76. [Google Scholar]