Abstract
GABA is a non-protein amino acid that accumulates rapidly in plant tissues in response to biotic and abiotic stress. There have been a number of suggestions as to the role that GABA might play in plants, ranging from a straightforward involvement in N metabolism to a signal mediating plant-animal and plant-microbe interactions. It has also been several proposed that it might function as an intracellular signalling molecule in plants. Here, we discuss recent evidence that plant cells respond at the molecular level to the presence of applied GABA. We argue that these data might serve as the basis for investigating the possible signalling role for GABA in plant development and stress responses in more detail.
Key Words: 14-3-3 proteins, GABA, signalling, gene expression, stress, senescence
GABA (γ-aminobutyric acid), which comprises a significant fraction of the free amino acid pool in plant cells, was first identified in potato tubers over half a century ago, but its functions remained obscure for many years. In animal systems, GABA is present at high levels in the brain where it acts as an important neurotransmitter. GABA is synthesised in a pathway known as the GABA shunt, which operates not only in the animals, but in bacteria, fungi and plants too.1,2 The function of GABA in plants has attracted renewed attention in the last decade following the discovery that intracellular and/or extracellular GABA concentrations increase rapidly in response to a range of stresses. Subsequently, a number of possible roles for GABA and the GABA shunt in plants have been suggested.1,2 These include acting as a buffering mechanism in C and N metabolism, cytosolic pH regulation, protection against oxidative stress and defence against herbivorous pests. It has been proposed recently that one common function of GABA might be to mediate interactions between plants and other organisms, including bacterial and fungal pathogens, nematodes and insect pests.3 On the other hand, because of the rapid increases in GABA concentration in response to stress, it has sometimes been inferred that GABA might act as an intracellular signalling molecule in plants. However, few studies have adopted molecular approaches to GABA function, and molecular responses are largely unknown.
Evidence for GABA Signalling in Plants
Perhaps the clearest evidence for GABA signalling in plants comes from the characterisation of an Arabidopsis GABA transaminase mutant (pop2) which revealed that a GABA concentration gradient in the style is required to guide pollen tubes to the ovary.4 The effect of disrupting GABA metabolism on plant development is also suggestive of more than a metabolic role: the over-accumulation of GABA that resulted from ectopic expression of constitutively actived glutamate decarboxylase in transgenic tobacco plants was correlated with developmental abnormalities.5 Both GABA and glutamate (at 5 or 10 mM) stimulated growth of Lemna, while other amino acids had no effect.6 Further evidence that exogenous GABA has specific effects that extend beyond its role as a N source has recently emerged from gene expression studies. In roots of Brassica napus, a 100 µM GABA treatment induced a significant increase in abundance of mRNA for the BnNRT2 nitrate transporter, while other amino acids either had no effect or (in the case of Gln) had the opposite effect.7 Most recently, we have published results showing that in the presence of high concentrations of external calcium, GABA, but not glutamate, strongly down-regulates seven of the nine 14-3-3 genes expressed in Arabidopsis seedlings, an effect that was also dependent on ethylene and abscisic acid signalling.8 One hypothesis is that calcium and GABA interact to signal specific forms of environmental stress. Alternatively, it is possible that GABA might act as signal for N status in germinating seedlings, in much the same way that sugars signal for C status.
Other Molecular Targets for GABA
Following this discovery, we wanted to see whether the expression of other genes might be regulated by exposure to GABA. Using RNA isolated from seedlings grown on 10 µM GABA as before,8 we analysed global changes in gene expression using an Affymetrix ATH1 GeneChip™ microarray. In comparison with seedlings grown in the absence of GABA, we identified 71 genes that were downregulated and 36 genes that were upregulated by GABA, using a 2.5-fold difference in probe signal to define the altered gene set. Changes in expression of a number of these genes was confirmed by RT-PCR. Although this was a preliminary, unreplicated experiment, a bioinformatic analysis of the data revealed several intriguing aspects of the GABA response. Of the genes downregulated by GABA, 90% are expressed exclusively or preferentially in roots, according to data in the Genevestigator microarray database.9 This contrasts with the random distribution in different tissues and organs of the basal expression of GABA-induced genes. Furthermore, there is a rather striking abundance of genes encoding proteins associated with the plant cell wall and extracellular matrix. Iterative group analysis10 identified peroxidases (10/71 downregulated genes) and lipid transfer proteins (6/71 downregulated genes), as highly significantly over-represented, and a number of other genes encoding cell wall proteins such as arabinogalactan proteins (five genes), hydroxyproline-rich glycoproteins and others, are also down-regulated by GABA. On the basis of our gene expression data, we were interested to know whether application of external GABA might affect root growth. Hence, we measured the length of the primary roots of Arabidopsis seedlings grown on media containing 1 mM total N with the addition of either 10 mM GABA, 10 mM Glu. The results clearly indicate a significant reduction in root growth in the presence of GABA, but not Glu (Fig. 1). It is tempting to speculate, therefore, that the changes in gene expression indicated by the microarray are manifest in the plant as reduced root growth.
Figure 1.
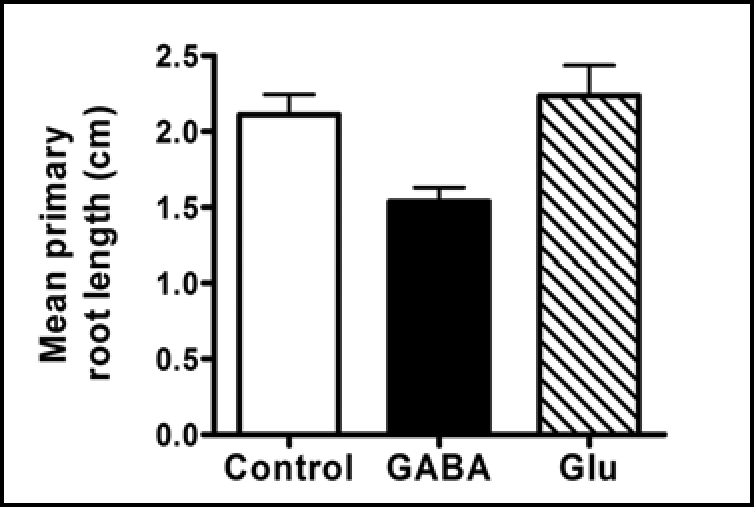
GABA inhibits Arabidopsis root growth. Histogram showing mean primary root lengths of 7-day-old Arabidopsis seedlings grown on vertical agar plates containing a modified Gamborg's B5 medium16 with the addition of either 10 mM GABA or glutamate (Glu), or with no supplementary amino acids (control). Error bars represent SEM. Nitrate was included in the medium in the form of 0.33 mM NH4NO3 (1 mM total N) to suppress the inhibition of root growth by Glu reported by Walch-Liu et al.17
The GABA upregulated gene set by contrast, showed a high degree of overlap (24/36 genes) with genes upregulated during senescence in tissue cultured cells. Interestingly, the Genevestigator database also revealed that both sets of GABA-regulated genes may also be regulated by cytokinin, a hormone intimately involved with senescence. Twenty nine out of 36 GABA upregulated genes were upregulated by application of 6-benzyl adenine to Arabidopsis seedlings, whilst 36 GABA downregulated genes were downregulated by the same treatment. A large proportion of the GABA upregulated genes also appear to be downregulated by glucose addition (33/36 genes) and by nitrate starvation (29/36 genes). Earlier studies also linked GABA with senescence, including observations of increased GABA concentrations11,12 and GABA-transaminase expression13 in senescent leaves. Together, these correlations begin to suggest a possible role for GABA in C and N metabolism both in post-germinative growth and senescence.
Whilst these data do not provide any direct evidence for a role for GABA in plant cell signalling, our published and preliminary unpublished results provide a platform for a more detailed analysis of the molecular and physiological effects of GABA in plants. We also recognise the limitations of the experimental system that we have used to date, and it will be important to examine the kinetics and dose responses of GABA-induced changes in gene expression. Together with the availability of additional tools such as mutants in GABA biosynthetic genes (e.g., ref. 14) and GABA catabolic enzymes (e.g., ref. 15), these ideas present a range of opportunities to begin to reveal the elusive functions of GABA in plants.
Acknowledgements
The author acknowledges the contributions of Dr. Muriel Lancien and the several undergraduate students who helped to generate the data discussed in this article, and Professor Brian Forde for his helpful discussions.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4335
References
- 1.Bouché N, Fromm H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004;9:110–115. doi: 10.1016/j.tplants.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Shelp BJ, Bown AW, McLean MD. Metabolism and functions of gamma-aminobutyric acid. Trends Plant Sci. 1999;4:446–452. doi: 10.1016/s1360-1385(99)01486-7. [DOI] [PubMed] [Google Scholar]
- 3.Shelp BJ, Bown AW, Faure D. Extracellular γ-aminobutyrate mediates communication between plants and other organisms. Plant Physiol. 2006;142:1350–1352. doi: 10.1104/pp.106.088955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palanivelu R, Brass L, Edlund AF, Preuss D. Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell. 2003;114:47–59. doi: 10.1016/s0092-8674(03)00479-3. [DOI] [PubMed] [Google Scholar]
- 5.Baum G, LevYadun S, Fridmann Y, Arazi T, Katsnelson H, Zik M, Fromm H. Calmodulin binding to glutamate decarboxylase is required for regulation of glutamate and GABA metabolism and normal development in plants. EMBO J. 1996;15:2988–2996. [PMC free article] [PubMed] [Google Scholar]
- 6.Kinnersley AM, Lin F. Receptor modifiers indicate that γ-aminobutyric acid (GABA) is a potential modulator of ion transport in plants. Plant Growth Reg. 2000;32:65–76. [Google Scholar]
- 7.Beuve N, Rispail N, Laine P, Cliquet JB, Ourry A, Le Deunff E. Putative role of γ-aminobutyric acid (GABA) as a long-distance signal in up-regulation of nitrate uptake in Brassica napus L. Plant Cell Environ. 2004;27:1035–1046. [Google Scholar]
- 8.Lancien M, Roberts MR. Regulation of Arabidopsis thaliana 14-3-3 gene expression by γ-aminobutyric acid. Plant Cell Environ. 2006;29:1430–1436. doi: 10.1111/j.1365-3040.2006.01526.x. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W. Genevestigator: Arabidopsis microarray database and analysis toolbox. Plant Physiol. 2004;136:2621–2632. doi: 10.1104/pp.104.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breitling R, Amtmann A, Herzyk P. Iterative Group Analysis (iGA): A simple tool to enhance sensitivity and facilitate interpretation of microarray experiments. BMC Bioinformatics. 2004;5:34. doi: 10.1186/1471-2105-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz C, Purdy S, Christ A, Morot-Gaudry JF, Wingler A, Masclaux-Daubresse C. Characterization of leaf markers to determine the extent and variability of leaf senescence in Arabidopsis: A metabolic profiling approach. Plant Physiol. 2005;138:898–908. doi: 10.1104/pp.105.060764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masclaux C, Valadier MH, Brugiere N, Morot-Gaudry JF, Hirel B. Characterization of the sink/source transition in tobacco (Nicotiana tabacum L.) shoots in relation to nitrogen management and leaf senescence. Planta. 2000;211:510–518. doi: 10.1007/s004250000310. [DOI] [PubMed] [Google Scholar]
- 13.Ansari MI, Lee RH, Chen SCG. A novel senescence-associated gene encoding γ-aminobutyric acid (GABA): Pyruvate transaminase is upregulated during rice leaf senescence. Physiol Plant. 2005;123:1–8. [Google Scholar]
- 14.Bouché N, Fait A, Zik M, Fromm H. The root-specific glutamate decarboxylase (GAD1) is essential for sustaining GABA levels in Arabidopsis. Plant Mol Biol. 2004;55:315–325. doi: 10.1007/s11103-004-0650-z. [DOI] [PubMed] [Google Scholar]
- 15.Bouché N, Fait A, Bouchez D, Moller SG, Fromm H. Mitochondrial succinic-semialdehyde dehydrogenase of the γ-aminobutyrate shunt is required to restrict levels of reactive oxygen intermediates in plants. Proc Natl Acad Sci USA. 2003;100:6843–6848. doi: 10.1073/pnas.1037532100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gamborg OL, Miller RA, Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968;50:151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- 17.Walch-Liu P, Liu LH, Remans T, Tester M, Forde BG. Evidence that L-glutamate can act as an exogenous signal to modulate root growth and branching in Arabidopsis thaliana. Plant Cell Physiol. 2006;47:1045–1057. doi: 10.1093/pcp/pcj075. [DOI] [PubMed] [Google Scholar]


