Abstract
Over decades, the so-called growth regulator or auxin herbicides had resisted all efforts to elucidate their molecular interactions and the biochemical and physiological basis of their phytotoxicity.1–3 The identification and crystal structure analysis of receptors for auxin perception4–8 and the discovery of a new hormone interaction in signalling between auxin, ethylene and the up-regulation of 9-cis-epoxycarotenoid dioxygenase (NCED) in abscisic acid (ABA) biosynthesis,9 leading to ABA accumulation,3 are long steps towards understanding of auxin herbicide action in dicot plants.
Key Words: abscisic acid, auxin herbicides, auxin receptors, 9-cis-epoxycarotenoid dioxygenase, ethylene, gene expression, indole-3-acetic acid
With their application in crop production, auxin herbicides started a new era of weed control in modern crop production due to their systemic mobility in the plant and to their selective action, preferentially against dicot weeds in cereal crops.1–3 Auxin herbicides belong to different chemical classes, which include phenoxycarboxylic acids, benzoic acids, pyridine- carboxylic acids, aromatic carboxymethyl derivatives and quinolinecarboxylic acids. The essential structural requirement for their activity is a strong negative charge on the carboxyl group of the dissociated molecule, which is separated from a weaker positive charge on the planar aromatic ring with a distinct distance.1–3
Auxin herbicides mimic the Janus face action of the main auxin indole-3-acetic acid (IAA) in higher plants.1–3 However, they are long-lasting, particularly due to their higher stability in the plant, and, therefore, more effective than IAA. Auxin herbicides stimulate a variety of growth and developmental processes when present at low concentrations at the cellular sites of action. However, with increasing concentration and auxin activity in the tissue, growth is disturbed and the plant is lethally damaged.1–3
From the molecular view, extensive perception by auxin receptor(s) or subsequent signal transduction processes were assumed to be the primary mechanism of herbicide action as a consequence of supraoptimal endogenous auxin concentrations.1–3
With the recent discovery of transport inhibitor response 1 (TIR1)-type auxin receptors, a perception mechanism has been identified which could account for a large part of the repertoire of auxin-mediated responses.4,6 The F-box protein TIR1 is the recognition component of a Skp1-cullin-F-box (SCF)-type ubiquitin-protein ligase (SCFTIR1) which is part of the ubiquitin-proteasome pathway for protein degradation.4,6 The substrates for TIR1, Aux/IAA transcriptional repressor proteins, are recruited to TIR1 in an auxin-dependent manner and, after binding to TIR1, are degraded.4,6,10 Crystallographic studies revealed that IAA binds to the base of the same TIR1 pocket that docks the Aux/IAA substrate on top of IAA which occupies the rest of the TIR1 pocket.8 IAA appears to function as a “molecular glue” to enhance TIR1-Aux/IAA protein interaction.8 This results in subsequent ubiquitination of Aux/IAA proteins, marking them as substrates for proteasomal degradation.4,6,8 The loss of Aux/IAA repressor proteins leads to derepression of preexisting DNA-binding transcriptional activator proteins, the auxin response factors, ARFs.10 Then, ARFs continuously activate transcription of auxin-response genes as long as auxin concentrations remain high.10 In accordance with the hypothesized action of auxin herbicides as synthetic mimics of IAA, TIR1 binds and functionally responds also to the auxin herbicide 2,4-dichlorophenoxy acetic acid (2,4-D) and to 1-naphthalene acetic acid (1-NAA).4,6 In addition, crystal structures of TIR1 in association with the auxin ligands IAA, 2,4-D and 1-NAA showed that IAA binds to a partially promiscuous site at the base of the TIR1 pocket, which can also accommodate the auxin analogous.8 IAA binds to TIR1 with the greatest affinity, and binding involves the side-chain carboxyl group as well as the ring system.8 The Arabidopsis genome encodes five homologs of TIR1.7 Three of them, the auxin signalling F-box proteins AFB1, AFB2 and AFB3, also mediate auxin response.5 Consequently, seedlings of the quadruple Arabidopsis mutant tir1, afb1, afb2, afb3 are auxin insensitive and exhibit severe developmental and morphological phenotypes.5 This suggests that the four homologs collectively modulate the plant response to IAA and 2,4-D.5 In addition, mutations in the TIR1 homolog AFB5 have been found to confer resistance particularly to auxin herbicides from pyridinecarboxylic acid type, with only minimal cross-resistance to 2,4-D or IAA.7 This indicates that chemical specificity to the different classes of auxin herbicides can be mediated principally by different auxin receptor proteins.7 Future research should explore the role of TIR1/AFB receptors in perceiving the chemically diverse signals of auxin herbicides in more detail and in mediating the spectrum of biological activities dependent on the sensitivity of tissue, physiological stage and plant biotype and species. These studies should also give us an answer whether the TIR1/AFB receptor family accounts for all auxin activities or additional signalling pathways, possibly via auxin binding protein 1 (ABP1) are involved.11
Overall, the TIR1/AFB receptors could link binding of auxin herbicides or IAA at supraoptimal concentrations directly to transcription factor abundance and over-expression of auxin-responsive genes that in turn leads to the succeeding series of biochemical and physiological events associated with the herbicide action. Excessive stimulation of ethylene production through induced 1-aminocyclopropane-1-carboxylic acid (ACC) synthase in biosynthesis is a well-known early event of auxin herbicides.2,3,12 Isoforms of ACC synthase genes (ACS) were shown to be differentially expressed or post-transcriptionally or post-translationally regulated by auxins within a few minutes of treatment.13 The resulting ethylene burst causes growth abnormalities and senescence.2,3,12
A more important factor implicated in growth inhibition and the actual phytotoxic response to auxins is the overproduction of ABA.3,14,15 In previous work, induction of ABA accumulation was demonstrated for auxin herbicides from the different chemical classes and IAA in a variety of dicot species.3 Exemplified first for the highly-sensitive dicot cleavers (Galium aparine), auxins trigger de novo ABA biosynthesis.15 Analysis of pathway intermediates revealed that ABA biosynthesis is exclusively induced in the shoot tissue by increasing xanthophyll cleavage, leading to increased production of the ABA precursor xanthoxin.15 This key regulated step in the pathway is catalysed by the plastid enzyme NCED, which is encoded by a family of NCED genes.16 The enzymatic activity of NCED can not be measured up to now.16 Nevertheless, NCED was assumed to be regulated by auxin treatment because work had additionally excluded that enhanced NCED precursor supply, steps in the pathway after NCED, or reduced ABA degradation and conjugation did contribute to ABA accumulation.15 Moreover, studies using inhibitors of biosynthesis and tomato mutants defective in perception or synthesis of ethylene or ABA suggested that auxin-stimulated ethylene is the main trigger for ABA accumulation.3,14,15 In accordance, transcriptome analysis in 2,4-D treated Arabidopsis showed expression of NCED1 and genes involved in ethylene signalling and biosynthesis.17
Recently, a gene (GaNCED1) from Galium shoot tissue was cloned based on sequence similarity to known NCED genes from different species.9 This made it possible to follow the time-course of both auxin-induced NCED gene expression and biosynthesis of ethylene and ABA.9 Within one hour of treatment of Galium plants, root-applied IAA and auxin herbicides led to transient increases in GaNCED1 mRNA levels exclusively in the shoot tissue.9 Three hours after treatment, transcript levels increased to maximum values 40-fold greater than in controls; thereafter the level declined.9 During this time, the water content and the osmotic potential in Galium shoot tissue were not changed, which indicates that GaNCED gene expression was not upregulated by auxin-mediated turgor changes. ABA began to increase with a lag phase of two hours and reached levels 24-fold higher than those in controls after 24 hours.9 Interestingly, increases in GaNCED1 mRNA preceded those in ACC synthase activity, ACC and ethylene production. This indicates that ethylene or ACC are not the primary triggers for gene activation of NCED.9 In accordance, applied inhibitors of ethylene biosynthesis only slightly affected the increase in GaNCED1 transcript levels by auxin.9 Nevertheless, ethylene inhibitors considerably decreased auxin-induced ABA accumulation, indicating that auxin-induced ethylene is required for ABA accumulation, but plays only a minor role in NCED gene expression.9
In conclusion, these results suggest that besides their stimulatory effects on gene expression in ethylene and gibberellin biosynthesis,3,18 auxin herbicides and IAA are able to directly trigger gene activation of NCED, which in turn is required for up-regulation of ABA biosynthesis (Fig. 1). Future research has to clarify if auxin signalling in NCED gene expression also involves SCFTIR1 mediated degradation of transcription repressors, such as Aux/IAA (Fig. 1). Likewise, it should be investigated in more detail if induction of NCED gene expression is an ubiquitous effect of auxins in susceptible plants. This is suggested because auxin-induced ABA accumulation has been observed in a variety of dicot species.3 In addition, downstream of gene expression, NCED activity appears to be stimulated by auxin-induced ethylene, leading to lasting ABA biosynthesis (Fig. 1). Ethylene-mediated up-regulation of NCED activity could include increasing synthesis, activity and/or stability of the enzyme protein. However, no information is available so far, how an ethylene signalling process regulates NCED activity post-transcriptionally. Accumulated ABA in the shoot tissue is translocated within the plant and mediates events in the auxin herbicide syndrome, such as growth inhibition, tissue decay and plant death3 (summarized in Fig. 1).
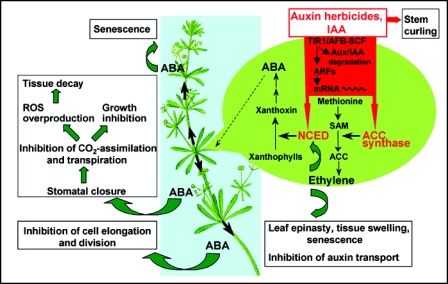
Figure 1.
Proposed mechanism and mode of action of auxin herbicides and the phytohormone indole-3-acetic acid (IAA) at supraoptimal endogenous concentrations in dicot plant species, as illustrated for cleavers (Galium aparine). Auxin herbicides are perceived by a small family of F-box proteins including the transport inhibitor response 1 (TIR1) and homolog auxin signalling F-box (AFB) proteins. Auxin binding to the TIR1/AFB auxin receptors tethers transcriptional repressor proteins, such as Aux/IAA, to a Skp1-cullin-F-box (SCF) ubiquitin-protein ligase for proteasomal degradation. The loss of Aux/IAA repressors leads to derepression of transcriptional activator proteins, the auxin response factors (ARFs), which activate transcription of auxin-response genes. In the shoot tissue, particularly genes of 1-aminocyclopropane-1-carboxylic acid (ACC) synthase in ethylene and 9-cis-epoxycarotenoid dioxygenase (NCED) in abscisic acid (ABA) biosynthesis are overexpressed. Produced ethylene elicits the downward curvature of leaves (leaf epinasty) and tissue swelling, and regulates auxin levels locally through inhibition of auxin transport. Concomitantly, auxins lead to horizontal stem curvature (stem curling). Ethylene also stimulates NCED activity post-transcriptionally, leading to lasting ABA biosynthesis. NCED catalyses xanthophyll cleavage, leading to increased production of xanthoxin and ABA. ABA is distributed within the plant and mediates stomatal closure which limits transpiration and carbon assimilation, accompanied by an overproduction of reactive oxygen species (ROS). In addition, ABA directly inhibits cell division and expansion and promotes, together with ethylene, foliar senescence with chloroplast damage and destruction of membrane and vascular system integrity. Growth inhibition, tissue desiccation and decay and finally plant death are the consequences. SAM, S-adenosylmetionine.
Acknowledgements
Thanks are due to Dr. Bryan Speakman for critical reading of the English manuscript.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4417
References
- 1.Cobb A. Herbicides and Plant Physiology. Chapman Hall; 1992. Auxin-type herbicides; pp. 82–106. [Google Scholar]
- 2.Sterling TM, Hall JC. Mechanism of action of natural auxins and the auxinic herbicides. In: Roe RM, et al., editors. Herbicide Activity: Toxicology, Biochemistry and Molecular Biology. Amsterdam: IOS Press; 1997. pp. 111–141. [Google Scholar]
- 3.Grossmann K. Mediation of herbicide effects by hormone interactions. J Plant Growth Regul. 2003;22:109–122. [Google Scholar]
- 4.Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- 5.Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann JS, Jurgens G, Estelle M. Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell. 2005;9:109–119. doi: 10.1016/j.devcel.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Kepinski S, Leyser O. The Arabidopsis TIR1 protein is an auxin receptor. Nature. 2005;435:446–451. doi: 10.1038/nature03542. [DOI] [PubMed] [Google Scholar]
- 7.Walsh TA, Neal R, Merlo AO, Honma M, Hicks GR, Wolff K, Matsumura W, Davies JP. Mutations in an auxin receptor homolog AFB5 and in SGT1b confer resistance to synthetic picolinate auxins and not to 2,4-dichlorophenoxyacetic acid or indole-3-acetic acid in Arabidopsis. Plant Physiol. 2006;142:542–552. doi: 10.1104/pp.106.085969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature. 2007;446:640–645. doi: 10.1038/nature05731. [DOI] [PubMed] [Google Scholar]
- 9.Kraft M, Kuglitsch R, Kwiatkowski J, Frank M, Grossmann K. Indole-3-acetic acid and auxin herbicides upregulate 9-cis- epoxycarotenoid dioxygenase gene expression and abscisic acid accumulation in cleavers (Galium aparine): Interaction with ethylene. J Exp Bot. 2007;58:1497–1503. doi: 10.1093/jxb/erm011. [DOI] [PubMed] [Google Scholar]
- 10.Hagen G, Guilfoyle T. Auxin-responsive gene expression: Genes, promoters and regulatory factors. Plant Mol Biol. 2002;49:373–385. [PubMed] [Google Scholar]
- 11.Badescu GO, Napier RM. Receptors for auxin: Will it all end in TIRs? Trends Plant Sci. 2006;11:217–223. doi: 10.1016/j.tplants.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Abeles FB, et al., editors. Ethylene in Plant Biology. Academic Press; 1992. [Google Scholar]
- 13.Chae HS, Kieber JJ. Eto brute? Role of ACS turnover in regulating ethylene biosynthesis. Trends Plant Sci. 2005;10:291–296. doi: 10.1016/j.tplants.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Grossmann K. The mode of action of auxin herbicides: A new ending to a long, drawn out story. Trends Plant Sci. 2000;5:506–508. doi: 10.1016/s1360-1385(00)01791-x. [DOI] [PubMed] [Google Scholar]
- 15.Hansen H, Grossmann K. Auxin-induced ethylene triggers abscisic acid biosynthesis and growth inhibition. Plant Physiol. 2000;124:1437–1448. doi: 10.1104/pp.124.3.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor IB, Mulholland BJ, Jackson AC, McKee JM, Hilton HW, Symonds RC, Sonneveld T, Burbidge A, Stevenson P, Taylor IB. Regulation and manipulation of the biosynthesis of abscisic acid., including the supply of xanthophylls precursors. J Plant Growth Regul. 2005;24:253–273. [Google Scholar]
- 17.Raghavan C, Ong EK, Dalling MJ, Stevenson TW. Regulation of genes associated with auxin, ethylene and ABA pathways by 2,4-dichlorophenoxy-acetic acid in Arabidopsis. Funct Integ Genom. 2006;6:60–70. doi: 10.1007/s10142-005-0012-1. [DOI] [PubMed] [Google Scholar]
- 18.Ross JJ, O'Neill DP, Wolbang CM, Symons GM, Reid JB. Auxin-gibberellin interactions and their role in plant growth. J Plant Growth Regul. 2002;20:346–353. doi: 10.1007/s003440010034. [DOI] [PubMed] [Google Scholar]