Abstract
The intimate arbuscular mycorrhiza (AM) association between roots and obligate symbiotic Glomeromycota (‘AM fungi’) ‘feeds’ about 80% of land plants. AM forming fungi supply land plants with inorganic nutrients and have an enormous impact on terrestrial ecosystems. In return, AM fungi obtain up to 20% of the plant-fixed CO2, putatively as monosaccharides. In a recent work we have reported the characterization of the first glomeromycotan monosaccharide transporter, GpMST1, and its gene sequence. We discuss that AM fungi might take up sugars deriving from plant cell-wall material. The GpMST1 sequence delivers valuable data for the isolation of orthologues from other AM fungi and may eventually lead to the understanding of C-flows in the AM.
Key Words: arbuscular mycorrhiza, Geosiphon symbiosis, monosaccharide transporter, hexoses
The arbuscular mycorrhiza (AM) as an outstanding terrestrial plant symbiosis directly and indirectly is a driver of most terrestrial ecosystems. It is formed by ∼80% of land plants and by obligate symbiotic fungi of the phylum Glomeromycota.1 The glomeromycotan fungi usually are called ‘arbuscular mycorrhizal (AM) fungi’, or ‘AMF’, and obviously play an enormous ecological (and economical) role. Most land plants and glomeromycotan fungi are ‘joint systems’, forming the intimate AM.2 By this fact, statements like that of the BEG (European Bank of Glomeromycota) committee (1993): “The study of plants without their mycorrhizas is the study of artefacts; the majority of plants, strictly speaking, do not have roots—they have mycorrhizas” were provoked. AM fungi supply the vast majority of land plants with inorganic nutrients, mainly phosphorous, but also nitrogen, trace elements, and water. In return, they obtain up to >20% of the photosynthetically fixed CO2 as carbohydrates from the plants.3 It was calculated that, each year, 5 billion tons of carbon are transferred from plants to fungi (and therefore partly get deposited in the soil) via the AM symbiosis. AM fungi therefore represent a large sink for atmospheric CO2 on our planet and play a role in C-deposition in the soil.
Studying the AM Symbiosis is Difficult
The ‘AM fungi’ (Glomeromycota) are multikaryotic, asexual and obligate symbionts. Each of these features makes certain molecular biological investigations difficult, the combination sometimes impossible. One main problem is that, in the symbiotic phase, the fungi live within the plant root cells and “clean” fungal tissue cannot be harvested for e.g., gene-expression studies. Physiology and genetics of the fungal nutrient transport systems in this life stage are largely unknown. The sequencing of the first AM fungal genome is in progress as a milestone for obtaining basic data, however, this project is delayed. Moreover, for the post-genomic era, targeted strategies are urgently needed to characterize the fungal transcriptome in the symbiotic stage (i.e., within the plant root). Even for Arabidopsis thaliana, which unfortunately is a non-AM forming plant and therefore not a good model for the life strategy of most plants with this respect, less then ¼ of the genes are functionally characterized. Such functional characterisations will become an important topic for future research. Regarding the uptake of the vast amounts of carbohydrates by AM fungi, nothing was known about carbohydrate transporters themselves, but it was shown by tracer studies and radiorespirometry that hexoses are taken up within the roots.4,5 The difficulties of isolating high quality fungal mRNA from AM roots, since plant mRNA is dominating, seems to be reflected by the fact that no AM fungal sugar transporter sequence was identified, despite its key-importance in the symbiosis.
In our study we followed an approach using the advantages of a peculiar symbiosis formed by a glomeromycotan fungus (Geosiphon pyriformis) with cyanobacteria (Nostoc punctiforme), the unique ‘Geosiphon-symbiosis’.6 The work shows, which potential lies in the use of this symbiosis as a model for the AM.7
The Geosiphon-Symbiosis Helps to Uncover Mechanisms in the AM
Why should we use the peculiar Geosiphon-symbiosis with cyanobacteria as a model for the AM? The answer is, that the obligate symbiotic fungal partner evolutionary and functionally is an ‘AM fungus’. It is phylogenetically embedded within the Archaeosporales, one of the four main clades of the biotrophic Glomeromycota.8,9 Therefore the ancestors of Geosiphon pyriformis putatively formed AM—and we have unpublished preliminary data that indicate that G. pyriformis in parallel to the cyanobacteria symbiosis forms AM. The Geosiphon-Nostoc symbiosis also shows many structural, functional and also ecological parallels to the AM - it represents an ‘AM symbiosis at the fungus—cyanobacterium level’.6 Regarding the symbiotic stage of G. pyriformis (the so called ‘bladder’, Fig. 1) it is the homologous stage to the intraradical situation in the AM, where the nutrient exchange processes in-between the partners take place at the symbiotic interface.10 This offers several advantages for investigating fundamental aspects like partner recognition, evolutionary aspects and nutrient exchange mechanisms.
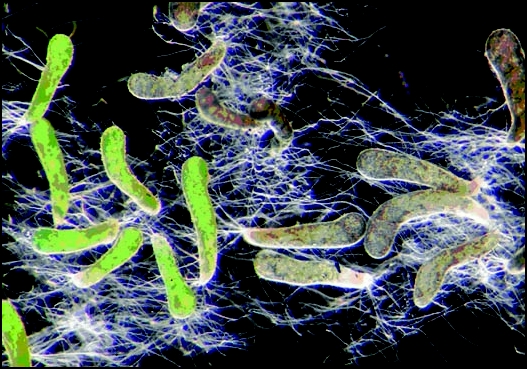
Figure 1.
Geosiphon pyriformis bladders in liquid medium, as they were used for mRNA isolation to construct the cDNA library (see text), the bladders shown are in average 1.5 mm in length and were harvested from cultures on sterilized, natural substrate. All attached hyphae were cut off, and bladders were incubated in liquid nutrient solution under illumination (light/dark cycles). All hyphae shown on the photograph were newly formed within the five days incubation in medium, so transcripts from actively growing hyphae are also represented in the cDNA library. The light green bladders are formed with a different cyanobacterial strain20 than the dark green bladders, which represent the type of color found in nature.
On the gene expression level, the clue of using the Geosiphon-symbiosis is that the fungal (eukaryotic) mRNA can be easily and specifically isolated out of the symbiotic stage with poly(A) discriminating methods, which were established to reproducibly isolate fungal mRNA from the symbiotic bladders. We constructed cDNA libraries suitable for yeast functional complementation from such mRNA11 and random sequencing of >100 cDNA clones did not result in any Nostoc sequences, indicating that the library is derived from nearly exclusively fungal transcripts.
The First Glomeromycotan Sugar Transporter
As reported in Schüßler et al. 2006, to which we address this addendum, high-quality fungal mRNA was isolated from symbiotic bladders and used to establish a cDNA yeast-expression library, which then served to isolate the fungal monosaccharide transporter GpMST1 gene by functional complementation of a yeast hexose transport null-mutant.12 GpMST1 has the highest affinities for glucose and mannose, followed by galactose and fructose. A KM of about 1.2 mM was determined for glucose. Since xylose is a main constituent of plant cell walls (see discussion below), we also tested whether it might be taken up by GpMST1. Indeed, xylose is indicated to slightly compete with glucose uptake and seems to be transported. This is now supported by unpublished results showing functional complementation of a hexose transport null-mutant yeast strain that is capable of xylose metabolism. For comparison it may be noted that in Saccharomyces cerevisiae the rate of xylose transport by hexose transporters corresponds to only 0.5% of glucose transport.13
Regarding the AM and Geosiphon symbioses, we hypothesize that GpMST1 is active at the symbiotic interface and therefore is located in the fungal symbiotic membrane. When interpreting the carbohydrate transport in the Geosiphon- and AM symbioses, it is crucial to know whether the transport is via facilitated diffusion or an active transport. 14C-glucose uptake (at pH 6.5) was very sensitive to protonophores and plasma membrane H+-ATPase inhibitors. The strong dependence on the presence of a proton gradient together with the pH dependence indicates that GpMST1 transport is mediated by secondary active proton cotransport.
Discussing C-Transport in AM
Nothing was known about sugar transporter genes in the AM. AM fungi seem to be restricted in their carbon supply since they nearly exclusively take up sugars via the symbiotic interface, that means from the photoautotrophic partner. Therefore it is conceivable that they might have a low number (maybe even only one?) of monosaccharide transporter genes for this purpose. We can not answer such a question yet, but our studies indicate that at least for GpMST1 there are no close paralogs, since PCR attempts using many different primer pairs always gave rise to GpMST1 amplicons only, with identical intron sequences.
Generally, the description of the first Glomeromycota monosaccharide transporter and its sequence opens the field for research on these key proteins, putatively being significant in the global C-flows. Isolation of orthologous genes from other AM fungi should now be relatively easy. Regarding GpMST1 itself, future tasks will be to isolate and characterize the promoter and one of the most important steps will be answering the question about where GpMST1 is ‘doing its job’, by localizing the gene product with antibodies or fusionproteins. Some indirect evidence already indicates that GpMST1 is indeed located at the symbiotic interface membrane. The membrane of the cup-shaped symbiosome compartment in Geosiphon is derived from the fungal plasma-membrane (by invagination) and retains the capability to synthesize chitin. This results in a thin cell wall layer within the symbiosome, ultrastructurally appearing like an arbuscule cell wall.10 The Geosiphon symbiosome membrane is a homologue of the arbuscular membrane in the AM, also showing the same function and bidirectional transport (in Geosiphon the cyanobacteria have to receive all P, water, etc., via the fungus, they do not have direct contact to the exterior). The fungal symbiotic membranes in the AM and Geosiphon symbioses show plasmamembrane features—but both membranes are much derived. In the case of the mature Geosiphon symbiosis the invaginated plasmamembrane probably has no contact to the ‘outer’ plasmamembrane any more. Our assumption, that GpMST1 is located at the perisymbiotic membrane is supported by the indirect evidence that no 14C glucose uptake could be shown for G. pyriformis bladders incubated in 14C glucose containing medium, a fact which was also observed for other AM fungi.4 GpMST1 potentially is the first characterised member of the ‘type’ of sugar transporters that is responsible for the transport of billions of tons of photosynthetically fixed CO2 from plants to AM fungi. Interestingly, the transporter sequence phylogenetically belongs to a new clade, comprising functionally noncharacterised putative transporter sequences, including many from parasitic fungi. Maybe this transporter-class is joined by some special features.
Where the Sugars Come From?
We are aware that this is still to some extend hypothetical, but if GpMST1 indeed represents the transporter type responsible for the monosaccharide uptake of AM fungi, what does it mean? Generally, symbiotically derived hexoses are interpreted as to be the exclusive energy source for glomeromycotan fungi,3,5 whereas some sugars presumably can also be taken up in nonsymbiotic stages.14 AM fungi, in contrast to the plant hosts, are limited in carbohydrates as energy source. This e.g., is reflected by retaining the C-skeleton of arginine when delivering nitrogen as NH4+ to the plant.15 Reasonably, the fungus should save valuable carbohydrates and a reasonable uptake mechanism would be by a non-energy-consuming process that e.g., could be facilitated diffusion. However, it is usually suggested that hexoses are taken up by active H+ cotransport and are derived from sucrose that is cleaved by apoplastic acid invertase into glucose and fructose. If the plant acidifies the perisymbiotic space, which is indicated,16 this would also be nonenergy-consuming for the fungus. One should add at this point, that the common plant sugar transport form, sucrose, may also serve as C-source in the Geosiphon-symbiosis, since the N. punctiforme symbiotic in Geosiphon produces high amounts of sucrose for osmoregulation.17,18
Regarding sugar transport, several indications, assumptions and facts may be incorporated into a working hypothesis regarding the general sugar uptake mechanisms of monosaccharides in the AM and Geosiphon-symbioses:
GpMST1 is probably located at the perisymbiotic membrane (homologous to arbuscule membrane). Its characterisation indicates that sugars are transported by means of H+-cotransport.
The symbiotic interface in the AM is acidic and acidified by the photoautotrophic partner, not by the fungus. Therefore the photoautotrophic partner might regulate hexose transport rates of the fungus by changing the apoplastic pH, maybe as a feedback mechanism dependent on the nutritional status.
The pH optimum of GpMST1 is, on the first view surprisingly, only slightly acidic. This could just be an effect of the heterologous yeast system, but it is also conceivable that this reflects the situation in the symbiosis. An only slightly acidic pH of 6–7 would be reasonable e.g., from the viewpoint, that a precipitation of the fungus-delivered phosphate within the perisymbiotic space would be avoided, and that a stronger acidification by the plant could diminish sugar uptake of the fungus, e.g., when arbuscules senesce and then get degraded by the plant.
GpMST1 effectively not only transports glucose, but also the other main cell wall constituent hexoses: mannose and galactose. Moreover it likely transports xylose.
These points may indicate that invertase cleaved sucrose is not the only source for the sugars taken up by AMF. H+-ATPase activity of the photoautotrophic partner might regulate the sugar supply by regulating acid invertase activity, the fungal transporter,19 but also by influencing the activity of (fungal?) hydrolases degrading cell-wall polysaccharides.
We therefore speculate that, in the AM as well as the AM-like Geosiphon-symbiosis, cell wall polysaccharides and glycoconjugates could be degraded in the symbiotic space, leading to a liberation of glucose, mannose, and galactose. These sugars might be used in parallel to sucrose derived glucose and fructose. It seems that xylose is also transported by GpMST1, and this might also hold true for arabinose—which would make such transporter types even interesting candidates for e.g., biofuel production from plant-residues, using genetically modified S. cerevisiae strains able to metabolize pentoses.
In general, we suppose that the Geosiphon symbiosis can and should be used to uncover key mechanisms in the AM and related symbioses, as exemplified by the characterisation of GpMST1. Such data then can be easily used as fundamental starting points for detailed studies in the AM.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/4465
References
- 1.Schüßler A, Schwarzott D, Walker C. A new fungal phylum, the Glomeromycota: Phylogeny and evolution. Mycol Res. 2001;105:1413–1421. [Google Scholar]
- 2.Smith SE, Read DJ. Mycorrhizal Symbiosis. London: Academic Press; 1997. [Google Scholar]
- 3.Bago B, Pfeffer PE, Abubaker J, Jun J, Allen JW, Brouillette J, Douds DD, Lammers PJ, Shachar-Hill Y. Carbon export from arbuscular mycorrhizal roots involves the translocation of carbohydrate as well as lipid. Plant Physiol. 2003;131:1496–1507. doi: 10.1104/pp.102.007765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfeffer PE, Douds DD, Jr, Bécard G, Shachar-Hill Y. Carbon uptake and the metabolism and transport of lipids in an arbuscular mycorrhiza. Plant Physiol. 1999;120:587–598. doi: 10.1104/pp.120.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solaiman Z, Saito M. Use of sugars by intraradical hyphae of arbuscular mycorrhizal fungi revealed by radiorespirometry. New Phytol. 1997;136:533–538. doi: 10.1046/j.1469-8137.1997.00757.x. [DOI] [PubMed] [Google Scholar]
- 6.Schüßler A, Wolf E. Geosiphon pyriformis - A glomeromycotan soil fungus forming endosymbiosis with cyanobacteria. In: Declerck S, Strullu DG, Fortin JA, editors. Soil Biology, Volume 4: In vitro culture of mycorrhizas. Berlin Heidelberg: Springer; 2005. pp. 271–289. [Google Scholar]
- 7.Schüßler A, Kluge M. Geosiphon pyriforme, an endocytosymbiosis between fungus and cyanobacteria, and its meaning as a model system for arbuscular mycorrhizal research. In: Hock B, editor. The Mycota IX. Berlin, Heidelberg, New York: Springer; 2001. pp. 151–161. [Google Scholar]
- 8.Schüßler A. Molecular phylogeny, taxonomy, and evolution of Geosiphon pyriformis and arbuscular mycorrhizal fungi. Plant Soil. 2002;244:75–83. [Google Scholar]
- 9.James TY, Kauff F, Schoch C, Matheny B, Hofstetter V, Cox C, Celio G, Guiedan C, Fraker E, Miadlikowska J, Lumbsch HT, Rauhut A, Reeb V, Arnold AE, Amtoft A, Stajich JE, Hosaka K, Sung GH, Johnson D, O'Rourke B, Crockett M, Binder M, Curtis JM, Slot JC, Wang Z, Wilson AW, Schüßler A, Longcore JE, O'Donnell K, Mozley-Standridge S, Porter D, Letcher PM, Powell MJ, Taylor JW, White MM, Griffith GW, Davies DR, Humber RA, Morton JB, Sugiyama J, Rossman AY, Rogers JD, Pfister DH, Hewitt D, Hansen K, Hambleton S, Shoemaker RA, Kohlmeyer J, Volkmann-Kohlmeyer B, Spotts RA, Serdani M, Crous PW, Hughes KW, Matsuura K, Langer E, Langer G, Untereiner WA, Lücking R, Büdel B, Geiser DM, Aptroot A, Diederich P, Schmitt I, Schultz M, Yahr R, Hibbett DS, Lutzoni F, McLaughlin DJ, Spatafora JW, Vilgalys R. Reconstructing the early evolution of the Fungi using a six-gene phylogeny. Nature. 2006;443:818–822. doi: 10.1038/nature05110. [DOI] [PubMed] [Google Scholar]
- 10.Schüßler A, Bonfante P, Schnepf E, Mollenhauer D, Kluge M. Characterization of the Geosiphon pyriforme symbiosome by affinity techniques: Confocal laser scanning microscopy (CLSM) and electron microscopy. Protoplasma. 1996;190:53–67. [Google Scholar]
- 11.Wipf D, Benjdia M, Rikirsch E, Zimmermann S, Tegeder M, Frommer WB. An expression cDNA library for suppression cloning in yeast mutants, complementation of a yeast his4 mutant and EST analysis from the symbiotic basidiomycete Hebeloma cylindrosporum. Genome. 2003;46:177–181. doi: 10.1139/g02-121. [DOI] [PubMed] [Google Scholar]
- 12.Wieczorke R, Krampe S, Weierstall T, Freidel K, Hollenberg CP, Boles E. Concurrent knock-out of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett. 1999;464:123–128. doi: 10.1016/s0014-5793(99)01698-1. [DOI] [PubMed] [Google Scholar]
- 13.Hamacher T, Becker J, Gardonyi M, Hahn-Hägerdal B, Boles E. Characterization of the xylose-transporting properties of yeast hexose transporters and their influence on xylose utilization. Microbiol. 2002;148:2783–2788. doi: 10.1099/00221287-148-9-2783. [DOI] [PubMed] [Google Scholar]
- 14.Hildebrandt U, Ouziad F, Marner FJ, Bothe H. The bacterium Paenibacillus validus stimulates growth of the arbuscular mycorrhizal fungus Glomus intraradices up to the formation of fertile spores. FEMS Microbiol Lett. 2006;254:258–267. doi: 10.1111/j.1574-6968.2005.00027.x. [DOI] [PubMed] [Google Scholar]
- 15.Govindarajulu M, Pfeffer PE, Jin H, Abubaker J, Douds DD, Allen JW, Bücking H, Lammers PJ, Shachar-Hill Y. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature. 2005;435:819–923. doi: 10.1038/nature03610. [DOI] [PubMed] [Google Scholar]
- 16.Gianinazzi-Pearson V, Arnould C, Oufattole M, Arango M, Gianinazzi S. Differential activation of H+-ATPase genes by an arbuscular mycorrhizal fungus in root cells of transgenic tobacco. Planta. 2000;211:609–613. doi: 10.1007/s004250000323. [DOI] [PubMed] [Google Scholar]
- 17.Knoblauch M. Vergleichende physiologische Charakterisierung von Nostoc punctiforme und Nostoc muscorum im Hinblick auf die Symbiose von Nostoc in Geosiphon pyriforme. In: Knoblauch M, editor. Master Thesis (in German) Frankfurt: University of Frankfurt, Department of Biology; 1995. pp. 1–70. (Ger). [Google Scholar]
- 18.Erdman N. Organic osmoregulatory solutes in blue-green algae. Z Pflanzenphysiol. 1983;110:147–155. (Ger). [Google Scholar]
- 19.van Kuyk PA, Jasper A, Maccabe AP, Hererro O, Ruijter GJG, Visser J. Aspergillus niger mstA encodes a high-affinity sugar/H+ symporter which is regulated in response to extracellular pH. Biochem J. 2004;379:375–383. doi: 10.1042/BJ20030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolf E, Schüßler A. Phycobiliprotein fluorescence of Nostoc punctiforme changes during the life cycle and chromatic adaptation: Characterization by spectral CLSM and spectral unmixing. Plant Cell Environ. 2005;28:480–491. [Google Scholar]