Abstract
Receptor-like kinases (RLKs) that function as pattern-recognition receptors (PRRs) play a key role in plant immune responses. The receptor recognizing flagellin in Arabidopsis, FLS2, is encoded by a membrane resident RLK. FLS2 is involved in preinvasive immunity against bacterial infection. Recent observations revealed that upon flagellin perception FLS2 accumulates in intracellular mobile vesicles and is then degraded. Reminiscent of ligand-induced receptor endocytosis in animals, FLS2 internalization is Wortmannin-sensitive. Mutation of the potentially phosphorylated residue threonine-867 impaired FLS2 endocytosis and flagellin-triggered responses. Furthermore, mutation of a PEST-motif abolished FLS2 endocytosis and downstream flagellin-elicited responses were affected. Thus, FLS2 endocytosis likely involves phosphorylation and ubiquitination events and appears to be interconnected with flagellin signaling. Similarly, TLR4, the mammalian PRR recognizing bacterial lipopolysaccharides (LPS) is internalized in a ligand specific manner. In this addendum, we discuss endocytic processes of plant RLKs focussing on FLS2 and provide a brief comparison with TLR4 endocytosis.
Key words: Endocytosis, RLK, FLS2, flagellin, TLR4, LPS
Introduction
Plant receptor-like kinases (RLKs) represent one of the largest protein families identified in Arabidopsis thaliana, with about ∼610 members.1 RLKs consist of an extracellular, a transmembrane and a cytoplasmic serine/threonine kinase domain. A major subgroup comprises RLKs containing leucine-rich repeats (LRRs) as extracellular domains and some of them have been shown to regulate diverse developmental processes. Few LRR-RLKs are reported to function as so-called pattern recognition receptors (PRRs) that mediate perception of pathogen-associated molecular patterns (PAMPs), which are conserved structures of whole classes of microbes. In mammals, the most important PRRs are the Toll-like receptors (TLRs).2 To date, the best characterized PRR in plants is the Arabidopsis flagellin sensing receptor kinase FLS2, which is discussed in further detail.3,4,5
FLS2—a Plant Receptor Kinase Recognizing Bacterial Flagellin
The receptor kinase FLS2 was identified in a screen for mutant plants that were insensitive to bacterial flagellin, the major component of the bacterial motility organ.3 Chinchilla et al. recently provided evidence for physical interaction between FLS2 and flg22, the elicitor active epitope corresponding to the most conserved domain of flagellin.6 The biological significance of the FLS2/flg22 pathway in plant immunity was shown by Zipfel et al.5 and further characterized by Melotto et al.7 Fls2 mutants are more susceptible than wild type plants when phytopathogenic bacteria were inoculated into the leaf surface.5 Perception of flg22 was found to induce closure of stomata, the entry sites for infections, and this may constitute to preinvasive immunity.7
In a recent report by Robatzek et al.,8 a functional fusion of FLS to the green fluorescent protein (GFP) was shown to localize strictly to cell membranes.8 However, upon addition of flg22 FLS2-GFP was rapidly and specifically internalized into mobile vesicles.8 Prolonged flg22 incubation resulted in a loss of FLS2-GFP signal indicating lysosomal and/or proteasomal degradation.8 Treatment with cytoskeleton inhibitors revealed a strongly reduced formation of flg22-induced FLS2-GFP vesicles.8 Furthermore, flg22-triggered FLS2 internalization was Brefeldin A (BFA) insensitive and Wortmannin-sensitive, providing evidence for an endocytic process.8
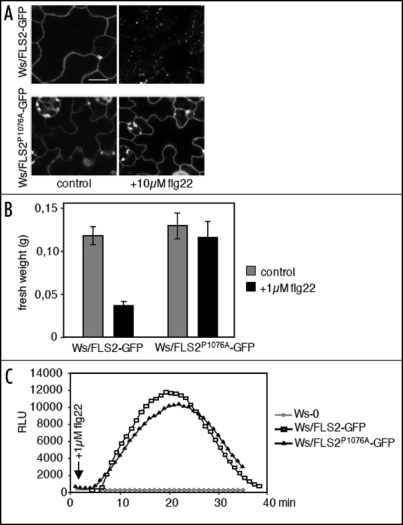
A key observation of this study is that flg22-induced FLS2-GFP internalization is blocked in the presence of kinase inhibitors.8 Following up the role of phosphorylation in FLS2 endocytosis, site-directed mutagenesis revealed a threonine residue within the juxta membrane region of FLS2 (T867) that when mutated rendered FLS2 impaired in internalization. In addition, flg22 responses are affected, suggesting a connection of endocytosis and signaling maybe involving endosome localized MAP kinases. Furthermore, Robatzek et al.8 reported that mutation of a PEST-like motif (P1076), which is implicated in monoubiquitin-triggered receptor endocytosis in yeast and animals,9 abolished FLS2 endocytosis (Fig. 1A). Similar to the FLS2T867V mutation, plants expressing the FLS2P1076A mutation were impaired in downstream flg22 signaling (Fig. 1B). Unlike the FLS2T867V mutant variant, the FLS2P1076A variant was found to still mediate flg22-triggered oxidative burst (Fig. 1C). This indicates an intimate interaction between FLS2 endocytosis and signaling not affecting early flg22-triggered responses. However, further investigations need to be done to distinguish the contribution of FLS2 endocytosis on various aspects of flg22 signaling and the point of convergence.
Figure 1.
Effect of the P1076A mutation on FLS2 endocytosis and flg22 signaling. (A) Subcellular localization of FLS2-GFP and FLS2P1076A-GFP expressed in the naturally occurring A. thaliana Ws-0 fls2 mutant in untreated or flg22 stimulated lines; scale bar 20 µM. (B) Seedling growth and (C), oxidative burst measured by luminescence of Ws/FLS2-GFP and Ws/FLS2P1076A-GFP lines in response to flg22.
Several reports demonstrate the internalization of plant LRR-RLKs involved in growth and development. The membrane resident RLK mediating physical interaction to the phytohormone brassinosteroid, BRI1, was shown also to localize to endosomes.10 This constitutive endocytosis is likely driven by endogenous brassinosteroid, which could not be further stimulated by exogenous applied brassinosteroid.
The LRR-RLK coreceptor of BRI1, BAK1 (also known as SERK3), forms heterodimers with BRI1 upon brassinosteroid perception and accelerates BRI1 endocytosis.10 SERK1, another member of the BAK1 family involved in somatic embryogenesis, is also subjected to endocytosis. However, this occurs only in the presence of the kinase-associated protein phosphatase (KAPP), a protein found to interact with many RLKs.11 To date, heterodimerization with a coreceptor could be only shown for BRI1 and this is related to regulatory processes of animal receptor tyrosine kinase (RTK) endocytosis.12
Induced Endocytosis of Immune Receptors
In mammals, pattern recognition is mediated by Toll-like receptors (TLRs), transmembrane proteins lacking a kinase domain. Ligand-induced endocytosis was recently demonstrated for TLR4 upon perception of bacterial lipopolysaccharides (LPS).13 Husebye et al found increased LPS signaling when TLR4 endocytosis was impaired and observed LPS-triggered TLR4 ubiquitination.13 Therefore, TLR4 endocytosis is involved in attenuation of LPS signaling.13 The authors discuss the role of several tyrosine-based tretrapeptide YxxΨ motifs, that have been identified as endocytic signatures.14 In mammals bacterial flagellin is recognized by TLR5.15 However, it is not yet known whether TLR5 is as well internalized.
Recently, the LRR-RLK EFR responsible for recognizing the bacterial elongation factor EF-Tu was identified.16 EFR contains the motif YxxΨ indicating that EFR is endocytosed just like FLS2. However, since FLS2 endocytosis likely involves the PEST-like motif, both endocytic pathways might differ. Functional relevance of the YxxΨ motif in plants was shown by Ron and Avni, who identified the xylanase receptor LeEix.17 Mutation of the YxxΨ motif rendered LeEix nonfunctional suggesting involvement of LeEix endocytosis in xylanase signaling.17
Conclusions and Perspectives
Receptor endocytosis in plants is a newly emerging field involving LRR-RLKs, which mediate plant growth, development and immunity. BRI1 and its coreceptor BAK1 heterodimerize and localize to both cell membranes and endosomes. Therefore, they exhibit similarities to RTKs that also require homo- or heterodimerization for signaling and are subjected to endocytosis. In contrast, membrane resident FLS2 only accumulates in endosomes upon induction by its ligand flagellin. FLS2 endocytosis likely depends on phosphorylation and ubiquitination events, and these will be further addressed in future. Regulatory domains involved in BRI1 and BAK1 endocytosis remain to be determined. Comparative studies of individual plant RLKs and animal PRR endocytosis might reveal similarities but also differences in distinct endocytic pathways.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=3594
References
- 1.Shiu SH, Karlowski WM, Pan R, Tzeng YH, Mayer KF, Li WH. Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell. 2004;5:1220–1234. doi: 10.1105/tpc.020834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasare C, Medzhitov R. Toll-like receptors: Linking innate and adaptive immunity. Microb Infect. 2004;6:1382–1387. doi: 10.1016/j.micinf.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Gomez-Gomez L, Bauer Z, Boller T. Both the extracellular leucine-rich repeat domain and the kinase activity of FLS2 are required for flagellin binding and signaling in Arabidopsis. Plant Cell. 2001;13:1155–1163. [PMC free article] [PubMed] [Google Scholar]
- 4.Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- 5.Zipfel C, Robatzek S, Navarro L, Oakeley E, Jones JDG, Felix G, Boller T. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature. 2004;428:764–767. doi: 10.1038/nature02485. [DOI] [PubMed] [Google Scholar]
- 6.Chinchilla D, Bauer Z, Regenass M, Boller T, Felix G. The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell. 2006;18:465–476. doi: 10.1105/tpc.105.036574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melotto M, Underwood W, Koczan J, Nomura K, He SY. Plant stomata function in innate immunity against bacterial invasion. Cell. 2006;126:969–980. doi: 10.1016/j.cell.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 8.Robatzek S, Chinchilla D, Boller T. Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes and Dev. 2006;20:537–542. doi: 10.1101/gad.366506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haglund K, Di Fiore PP, Dikic I. Distinct monoubiquitin signals in receptor endocytosis. Trends Biochem Sci. 2003;28:598–603. doi: 10.1016/j.tibs.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Russinova E, Borst JW, Kwaaitaal M, Cano-Delgado A, Yin Y, Chory J, de Vries SC. Heterodimerization and endocytosis of Arabidopsis brassinosteroid receptors BRI1 and AtSERK3 (BAK1) Plant Cell. 2004;16:3216–3229. doi: 10.1105/tpc.104.025387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah K, Russinova E, Gadella TW, Jr, Willemse J, De Vries SC. The Arabidopsis kinase-associated protein phosphatase controls internalization of the somatic embryogenesis receptor kinase. Genes Dev. 2002;16:1707–1720. doi: 10.1101/gad.220402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorkin A, Von Zastrow M. Signal transduction and endocytosis: Close encounters of many kinds. Nat Rev Mol Cell Biol. 2002;3:600–614. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- 13.Husebye H, Halaas O, Stenmark H, Tunheim G, Sandanger O, Bogen B. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 2006;25:683–692. doi: 10.1038/sj.emboj.7600991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurten RC. Sorting motifs in receptor trafficking. Adv Drug Deliv Rev. 2003;55:1405–1419. doi: 10.1016/j.addr.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Smith KD, Andersen-Nissen E, Hayashi F, Strobe K, Bergmann MA, Rassoulian Barrett SL, Cookson BT, Aderem A. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol. 2003;4:1247–1253. doi: 10.1038/ni1011. [DOI] [PubMed] [Google Scholar]
- 16.Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell. 2006;125:749–760. doi: 10.1016/j.cell.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 17.Ron M, Avni A. The receptor for the fungal elicitor ethylene-inducing xylanase is a member of a resistance-like gene family in tomato. Plant Cell. 2004;16:1604–1615. doi: 10.1105/tpc.022475. [DOI] [PMC free article] [PubMed] [Google Scholar]