Abstract
Ozone (O3)-induced cell death in two suspension-cultured cell lines of tobacco (Nicotiana tabacum L.) derived from Bel-W3 (hyper-sensitive to O3) and Bel-B (highly tolerant to O3) varieties were studied. By exposing the newly prepared cell lines to the pulse of ozonized air, we could reproduce the conditions demonstrating the difference in O3 sensitivity as observed in their original plants, depending on the exposure time. Since O3-induced acute cell death was observed in the dark, the requirement for photochemical reactions could be eliminated. Addition of several ROS scavengers and chelators inhibited the cell death induced by O3, indicating that singlet oxygen (1O2), hydrogen peroxide (H2O2), hydroxyl radical and redox-active metals such as Fe2+ play central roles in O3-induced acute damages to the cells. As expected, we observed the generation of 1O2 and H2O2 in the O3-treated cells using chemiluminescent probes. On the other hand, an NADPH oxidase inhibitor, superoxide dismutase (SOD), and some SOD mimics showed no inhibitory effect. Thiols added as antioxidants unexpectedly behaved as prooxidants drastically enhancing the O3-induced cell death. It is noteworthy that some ROS scavengers effectively rescued the cells from dying even treated after the pulse of O3 exposure, confirming the post-ozone progress of ROS-dependent cell death mechanism. Since one of the key differences between Bel-B and Bel-W3 was suggested to be the capacity for ROS detoxification by catalase, the endogenous catalase activities were compared in vivo in two cell lines. As expected, catalase activity in Bel-B cells was ca. 7-fold greater than that in Bel-W3 cells. Interestingly, Ca2+ chelators added prior to (not after) the pulse of O3 effectively inhibited the induction of cell death. In addition, increases in cytosolic Ca2+ concentration sensitive to Ca2+ chelators, ion channel blockers, and ROS scavengers were observed in the transgenic Bel-W3 cells expressing aequorin, suggesting the action of Ca2+ as a secondary messenger initiating the oxidative cell death. The O3-induced calcium response in Bel-W3 cells was much greater than Bel-B cells. Based on the results, possible pathways for O3-dependent generation of the lethal level of ROS and corresponding signaling mechanism for induction of cell death were discussed.
Key Words: calcium, cell death, Nicotiana tabacum L., ozone, reactive oxygen species
Introduction
Ozone (O3) produced by a complex series of photochemical reactions from primary precursor emissions of nitrogen oxides and volatile organic compounds, is a major secondary air pollutant often reaching high concentrations in urban areas under strong daylight, and studies are now suggesting that a steep increase in global background concentrations of O3 is in progress and thus the impact of atmospheric O3 to plants including valuable crops might be severer in the future world.1 Despite of great efforts to identify the physiological and biochemical elements of O3 tolerance in plants, the pictures obtained to date are not clear enough.2 The most widely accepted model describing the nature of O3 toxicity/tolerance is the oxidative stress model in which generation of reactive oxygen species (ROS) and release of oxidation products are involved in the generation and propagation of toxic compounds throughout the plants.2 However, this model is still incomplete and does not tell us which ROS is actually involved in the induced cell death in plants.
Several studies have shown that exposure to O3 elicits the production of ROS in plants. In order to understand oxidative signaling, the approaches to identify the chemical types of ROS and the subcellular site of ROS generation are of great importance.3 In 1990, an electron spin resonance (ESR) study has provided the first evidence for the presence of radical species in the leaves of O3-exposed plants such as pea (Pisum sativum L.) and bean (Phaseolus vulgaris L.) using N-t-butyl-α-phenylnitrone as a spin trap.4 However, the nature of the trapped radicals could not be identified. Later ESR study with replicated ESR signal subtraction methods using 1,1-diphenyl-2-picrylhydrazyl as a spin trap has shown that a signal with characteristics of superoxide (O2.-) could be generated in the leaves of bluegrass (Poa pratensis L.), ryegrass (Lolium perenne L.) and radish (Raphanus sativus L.) after being exposed to high concentrations (ca. 10–25 ppm) of O3.5 Since the detection of such signal was light-dependent, a role for chloroplasts as the sites of photochemical O2.- generation was suggested. Based on these ESR results, a model for O3 toxicity in plants has been proposed.5 According to this model, O3 approaches and penetrates into the cells across the plasma membrane and further reaches to the photosynthetic apparatus in the chloroplasts to participate in the photochemical reactions leading to generation of O2.-. As parts of the protective mechanism, the entry of O3 could be blocked by ascorbate in the apoplast and the released O2.- could be quenched by the chloroplastic superoxide dismutase (SOD)-peroxidase system. Thus only when excess, O3 can damage the cells via this pathway. However, actual role for O2.- in the O3-induced cell death has not been examined.
In general, short exposure to high concentration of O3 causes the formation of cell death lesions on the leaves of sensitive plants.6 Such localized cell death is a common feature of O3 phytotoxicity and is generally thought to be initiated by strong oxidizing action of O3 itself as well as by O3-derived ROS intermediates.7 Since early 1960s, Bel-W3, a tobacco (Nicotiana tabacum L.) variety hyper-sensitive to O3 which readily produces visually recognizable symptoms on leaves, has been used in many countries as an indicator of the presence of phytotoxic concentrations of O3.8 Bel-B tobacco is also widely used as the reference plant since this variety is highly tolerant to O3.8
In the present study, we studied the O3-induced cell death in suspension-cultured transgenic (expressing aequorin gene) and wild type cell lines of tobacco derived from Bel-W3 and Bel-B plants, by exposing the newly prepared cell lines to the pulse of ozonized air. Since these cell cultures lack endogenous chlorophylls, and the experiments were carried out in the dark, involvements of the reactions requiring the photo-dependently excited chlorophylls could be eliminated. Based on the cell biological and biochemical analyses of O3 toxicity, possible pathways for generating the lethal level of ROS from O3, and oxidative and calcium signal transduction pathways are proposed.
Materials and Methods
Chemicals.
Chemically synthesized coelenterazine was a gift from Prof. M. Isobe and Dr. M. Kuse (Nagoya University). Cypridina luciferin analog (CLA) was purchased from Tokyo Kasei Kogyo Co. (Tokyo, Japan). N-acetylcysteine and dimethylthi urea (DMTU) were obtained from Nacalai Tesque, Inc. (Kyoto, Japan). 1,2-bis(2-aminophenoxy)-ethane-N,N,N′,N′ tetraacetic acid (BAPTA) was purchased from Dojindo Laboratories, Inc. (Kumamoto, Japan). Hydroxyphenyl fluorescein and aminophenyl fluorescein were products of Daiichi Pure Chemicals Co. Ltd. (Tokyo, Japan). Diphenyleneiodonium chloride, Cu,Zn-SOD from bovine erythrocytes, 1,4-diazabicyclo[2.2.2]octane (DABCO), O,O′-bis(2-aminoethyl)ethyleneglycol-N,N,N′,N′-tetraacetic acid (EGTA), ethylenediaminetetraacetic acid iron(III) sodium salt (Fe(III)-EDTA), 4,5-dehydroxy-1,3-benzenedisulfonic acid disodium salt (Tiron), o-phenanthroline (o-Phe), Murashige-Skoog (MS) salts were obtained from Sigma (St. Louis, MO, USA). Trichloroacetic acid was from Katayama Chemical Industries Co., Ltd. (Osaka, Japan). Copper (II) salicylate was manually synthesized as described.9 Catalase from bovine liver, thiobarbituric acid (TBA), 2,2′-bipyridyl (Bipy), luminol, and other reagents of analytical grade were purchased from Wako Pure Chemical Industries (Osaka, Japan).
Plants.
For performance of O3-induced lesion formation on leaves (symptom reflecting the localized cell death), tobacco plants (Nicotiana tabacum L, cv. Bel-W3 and Bel-B) were grown in O3-free air under a 12/12 h light/dark regime (white fluorescent light, ca. 2000–5000 lx depending on the leaf positions) at 23 ± 1°C and 70 ± 5% relative humidity.
Preparation of Bel-W3 and Bel-B cell lines.
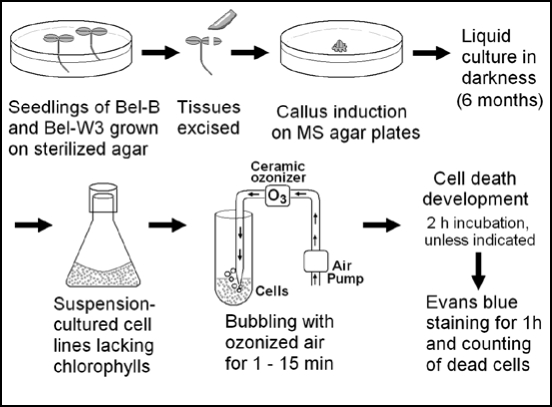
Cell suspension cultures derived from Bel-W3 and Bel-B tobacco plants were prepared as illustrated in Figure 1. For establishment of fresh cell lines derived from original plants, the seeds of Bel-W3 and Bel-B were sterilized in 1% (w/v) sodium hypochlorite and allowed to germinate on a sterilized MS agar plates lacking 2,4-dichlorophenoxy acetic acid (2,4-D). The seedlings were grown on the agar plates under a 12/12 h light/dark regime (white fluorescent light, ca. 2000–3000 lx) at 23 ± 1°C for three weeks. Then seedlings were harvested and the excised tissues were transferred onto MS agar medium containing 0.2 µg/ml of 2,4-D to promote the formation of callus. Suspension cultures of Bel-W3 and Bel-B cells were initiated by addition of cut pieces of the resultant calli of Bel-W3 and Bel-B to the MS liquid medium (pH 5.8) containing 0.2 µg/ml of 2,4-D. The cell suspension cultures (30 ml each in a 100 ml conical flask) were kept on gyratory shakers (at 130 rpm) at 23 ± 1°C in darkness, with occasional sub-culturing with 2 ml of confluent culture as inocula). After ca. six months of continuous propagation of the cells in MS liquid medium with constant sub-culturing (initially twice a month and later once a week), two stable cell lines, Bel-W3 and Bel-B were finally obtained.
Figure 1.
Preparation of cell lines derived from Bel-B and Bel-W3, and analysis of O3-induced cell death.
Since the cells were propagated in continuous darkness for more than half year, the original green color was completely lost (no recovery of chlorophylls was observed even after strong illumination with light, data not shown). Actually, no absorbance and fluorescence corresponding to chlorophylls was found in dimethylformamide extracts from the cell cultures while fluorescence from the tetrapyrrole intermediate-like compounds similar to chlorophyll metabolites10 showing excitation and emission maxima at around 330 and 420 nm, respectively, were found (data not shown). Therefore, the systems we used here could be successfully simplified since we can eliminate the involvement of chlorophylls as the catalysts of indirect photochemical processes leading to generation of O2•- from O3.5 Thus, more direct O3 toxicity in plant cells could be studied if we could reproduce the O3-induced cell death in these materials.
Transformation of the cells.
Agrobacterium-based transformation of tobacco suspension-cultured cells (cell lines, Bel-W3 and Bel-B) was carried out as described,11 using binary vectors encoding apoaequorin to be expressed in the cytosol.12 Following transformation, the cell lines overexpressing apoequorin were screened by monitoring the level of apoequorin expression with aequorin luminescence (ca. 466 nm; for details of luminescence measurements, see below sections) after addition of coelenterazine. The obtained transgenic cell lines were propagated as described for nontransgenic cells. Transformation was carried out at Graduate School of Medicine, Nagoya Univeristy, and all other processes were carried out at the Univeristy of Kitakyushu according to the Univeristy of Kitakyushu's GMO rules.
O3 treatments.
Ozone was generated by a ceramic ozonizer (NAVI Super Ceramics Ozonizer EO-mini, Kenis Kagaku Kyoeisha Ltd., Tokyo, Japan), equipped with an air pump (HiBlow 3EBS, Kenis Kagaku Kyoeisha Ltd., Tokyo, Japan). Leaf discs (Φ, 10 mm) were prepared from the fully expanded leaves (length, ≥200 mm) of tobacco plants with a cork borer. Leaf discs were placed on wet strips of Whatman paper in petri dishes without lids. The dishes with leaf discs were placed in a humidity-controlled fumigation chamber and exposed to a single pulse of 15 ppm O3 for certain length of time (0, 1, 2 and 4 h). By sampling the air in the fumigation chamber at the beginning and the end of each experiment, the actual concentration of atmospheric O3 surrounding the leaf discs in the chamber was monitored using O3 detection tubes (18M O3, Gastec Co., Kanagawa, Japan) and a gas sampling pump (GV-100S, Gastec Co., Kanagawa, Japan), and was adjusted to be 15 ppm.
For the O3 treatment of cell suspensions, ozonized air (1.5 L/min; 10 mg O3/h) was passed on the surface of the cell suspensions (10 ml) prepared from the three day-old cultures in 50 ml tubes (Fig. 1). By this way the cells could be exposed to the pulse of O3 for 1–15 min. Unless indicated, the pulse of O3 was applied to the cells in the dark to eliminate the effect of light.
Observation of lesions on the leaf discs.
Prior to observation of the symptom under a stereomicroscope, O3-treated or control air-exposed leaf discs were incubated in O3-free air for 24 h to allow the development of localized cell death. Lesions developed on the leaf discs were observed and photographed with a stereomicroscope equipped with a digital camera. For visualizing the lesions, the light source under the sample stage was used.
Determination of cell death.
To minimize the counts of old naturally dieing cells from the cell death analysis, both cell lines were conditioned as follows. The confluent cultures maintained with seven day-intervals of sub-culturing were used to inoculate the fresh MS liquid medium (2 ml culture to 30 ml medium) and precultured for three days. Then 6.5 ml of each preculture was transferred to 100 ml of fresh MS liquid medium (in a 500 ml conical flask) and further cultured for three days. These three day-old larger scale cultures were harvested and used for the experiments.
O3-induced cell death in the cell suspension culture was determined by staining the dead cells with Evans blue (0.1%, w/v) by mixing and incubating the cells and the dye for 1 h. Similarly to the O3-induced lesion development on leaf discs, certain length of incubation after the pulse of O3 exposure was required for induction of cell death (Fig. 1). Unless indicated, 2 h of incubation was employed prior to addition of the dye to the cells. Then stained cells were observed under microscopes (SMZ800 and Labophoto, Nikon, Tokyo, Japan; VHX-100, Keyence, Tokyo, Japan). For statistic analysis, 3–4 different digital images of cells under the microscope (each covering 50–100 cells to be counted) were acquired and stained cells were counted.
Catalase assay.
In vivo catalase activity in the tobacco cell suspension culture (defined as the activity to convert H2O2 to O2) was determined with a Clark-type oxygen electrode as reported.13 Cell cultures (3 day-old, 3 ml) were loaded into the vessel equipped with a Teflon-covered electrode and a micro magnetic stirrer, and covered with a lid with a pinhole. Then various amounts of H2O2 were injected with a micro-syringe through the pinhole and the H2O2 dose-dependent increase in dissolved O2 was recorded with a pen recorder.
Monitoring of ROS Production.
Generations of singlet oxygen (1O2) and O2•- were monitored by the chemiluminescence of CLA as described.13 The chemiluminescence of CLA specifically indicates the generation of O2•-, and of 1O2 to a lesser extent, but not of other ROS.14
Production of H2O2 was detected with the chemiluminescence of luminol as described.15 The chemiluminescences from CLA and luminol were measured using the CHEM-GLOW Photometer (American Instrument, Maryland, USA) equipped with a pen recorder and expressed as relative luminescence units (rlu).
Monitoring of [Ca2+]c.
The changes in [Ca2+]c in aequorin-expressing cell lines of Bel-B and Bel-W3 were monitored with the Ca2+-dependent emission of blue light from aequorin as previously described.14 The active form of aequorin was reconstituted by addition of 1 µM coelenterazine to the suspension culture of apoaequorin-expressing tobacco cells, 8 h prior to the measurements of [Ca2+]c. The aequorin luminescence was measured as described for chemiluminescence analyses.
Determination of lipid peroxide formation.
Since lipid peroxides are highly reactive with TBA, lipid peroxide formation was determined with TBA reactive substances (TBARS) assays. TBARS assays were performed on 0.2–1.0 ml of cell culture following the method of Buege and Aust.16 Two ml of TBA reagent prepared by dissolving 0.375% (w/v) TBA and 3 mM 2,6-di-tert-butyl-4 hydroxytoluene (BHT) in 15% (w/v) trichloroacetic acid and 22% (v/v) ethanol, was added to 1 ml of cell suspension. BHT was further supplemented (final conc., 0.5 mM). The samples in 15-mL centrifuge tubes were then heated to 100°C for 15 min, cooled down on ice, and spun down by centrifugation at 700 × g for 10 min. The absorbance of the supernatant was measured at 535 nm. The amount of malonaldehyde was calculated using an extinction coefficient of 1.56 × 105 M−1 cm−1.
Results
O3-sensitive and tolerant natures of original plants.
In order to study the mechanism of hypersensitivity and tolerance to O3 in suspension-cultured cells, we decided to confirm the background natures of plant materials first. Seedlings and leaf discs prepared from the fully expanded leaves of Bel-W3 and Bel-B plants were exposed to the pulse of ozonized air in a fumigation chamber maintaining high concentration of O3 (15 ppm) for 0, 1, 2 and 4 h. After 24 h of incubation in the O3-free air following O3 exposure, formation of lesions was examined under a microscope (Fig. 2). At this stage, formation of lesions was hardly recognized by eyes in either variety. However, when the leaf discs were illuminated from the bottom, early lesion formed on Bel-W3 leaf discs but not on Bel-B leaf discs could be visualized under a microscope. Without certain length of incubation after O3 exposure, such response reflecting the localized cell death in Bel-W3 leaf discs could not be detected (at least 8–12 h of incubation was required, data not shown), suggesting that the pulse of O3 exposure initiates the chains of biochemical reactions in the cells leading to active cell death development.
Figure 2.
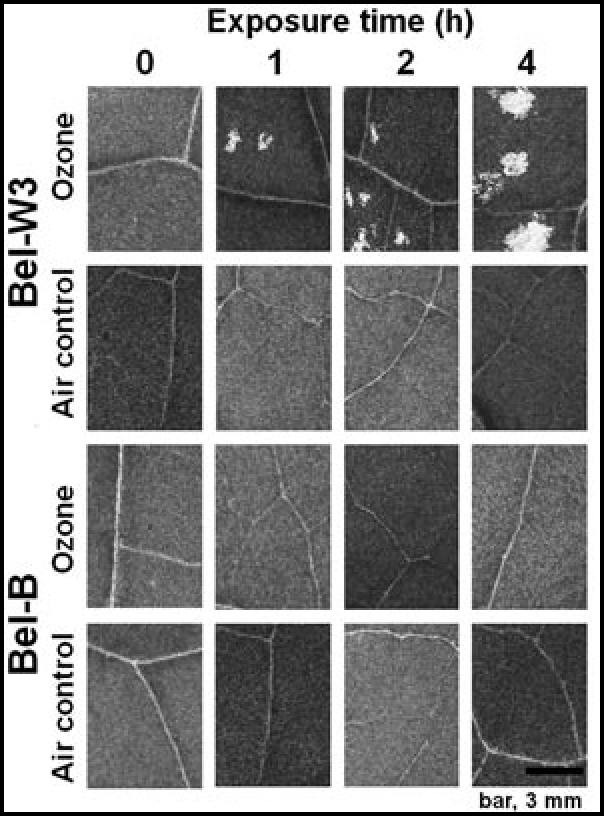
Development of O3-induced symptoms (lesions) on the leaf discs of Bel-W3 but not of Bel-B tobacco plants. Leaf discs (10 mm in diameter) were prepared from the fully expanded leafs (over 200 mm in length) of Bel-W3 and Bel-B plants with a cork borer. Leaf discs (ca. 200 discs for each variety) were exposed to 15 ppm O3 or control air in a humidity-controlled chamber for 0, 1, 2 and 4 h and further exposed to O3-free air for 24 h to allow the development of the induced lesions. Lesions on the discs were observed under a stereomicroscope equipped with a CCD-digital camera with illumination of light beneath the samples. Typical and representative images well reflecting the natures of two varieties are shown.
O3-induced cell death in suspension-cultured cells.
We studied the O3-induced cell death in two suspension-cultured cell lines derived from the pair of O3-sensitive and tolerant tobacco varieties, Bel-W3 and Bel-B. By exposing the newly prepared cell cultures (that have lost its chlorophylls during propagation in the dark) to the pulse of ozonized air (ca. 10 mg/h O3 flowing at 1.5 L/min), we could observe the induction of acute cell death determined with Evans blue staining (Fig. 3A) depending on the exposure time (Fig. 3B). Here, hypersensitiveness and tolerance of Bel-W3 cells and Bel-B cells, respectively, were successfully reproduced in the cell suspension cultures (Fig. 3B), as confirmed in the leaves of original plants.
Figure 3.
O3-induced cell death in suspension cultured cell lines derived from Bel-W3 and Bel-B tobacco varieties. (A) O3-induced cell death in suspension cultured tobacco cells. Cell cultures (totally 10 ml in 50 ml tube) were exposed to air (left) or ozonized air (right, ca. 10 mg O3/h flowing at 1.5 L/min) blowing over the culture medium. Then, aliquots (0.2 ml of cell suspension each) were harvested at 0, 1, 3, 4, 5, 6.5, 7, 8, 9, 10 and 15 min after initiation of O3 treatment (typical microscopic images at 15 min of exposure are shown). Each sample was kept static for 2 h at room temperature (23 ± 1°C) in a 1.5 ml plastic tube. Then dye was added and further incubated for 1 h and number of dead cells (stained cells) was counted under microscopes. Horizontal bar, 100 µm. (B) Effects of exposure time on the O3-induced cell death. Three randomly chosen digital images of different portion of each sample under microscopes were acquired dead (stained) cells were counted. Thus, each data point reflects the mean and S.E. (vertical bars, n = 3). Typical data from eight repeated experiments are shown here.
Effects of ROS scavengers.
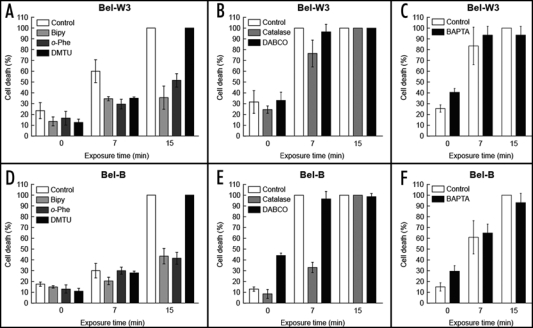
The advantage of the use of cell suspension instead of tissues or intact plants, is the ease of treatments with various biochemical inhibitors such as metal chelators and ROS scavengers. Five min prior to O3 exposure, the cell suspensions (both Bel-W3 and Bel-B) were treated with either of DABCO (10 mM), Bipy (2 mM), o-Phe (2 mM) or DMTU (50 mM). The O3-induced cell death in both cell lines was completely inhibited in the presence of DABCO which is a strong and selective scavenger of 1O2 (Fig. 4A and C). Therefore, involvement of 1O2 in O3 toxicity was suggested.
Figure 4.
Inhibition of O3-induced cell death in Bel-W3 and Bel-B tobacco cell lines by pretreatments with ROS scavengers and metal chelators. Cell death in Bel-W3 cells (A and B) and Bel-B cells (C and D) was induced by exposing the cells to ozonized air (for 0, 7, 15 min). For development of cell death, cells were incubated for further 2 h after O3 exposure. Prior to O3 exposures, cell suspension was treated with DABCO (10 mM), catalase (2000 units/ml), Bipy (2 mM), o-Phe (2 mM), DMTU (50 mM), or BAPTA (5 mM). Four randomly chosen digital images of different portion of each sample under microscopes were acquired and stained cells were counted. Thus, each data point reflects the mean and S.E. (vertical bars, n = 4). Typical data from three replications are shown.
Exogenous addition of bovine liver catalase (2000 units/ml) successfully rescued the Bel-W3 cells from dying when exposed to ozonized air for 7 min (Fig. 4E, left) or longer periods (8–10 min, data not shown). However, the cells exposed to O3 for 15 min could not be rescued by addition of catalase, indicating that more H2O2 over the capacity of added catalase was generated during the longer exposure to O3. In both Bel-W3 and Bel-B cell, addition of 20000 units/ml of catalase prior to O3 treatment successfully prevented the development of cell death even after 15 min-long exposure to O3 (Fig. 4E right). Protective action of catalase clearly suggested that H2O2 is involved in the O3-induced cell death in these cells.
In addition to H2O2, involvements of hydroxy radical (HO.) and iron (and/or copper) in the induced cell death were suggested since DMTU, a scavenger of HO., and o-Phe and Bipy, two chelators of iron (and copper), showed strong inhibition of the O3-induced cell death in both cell lines (Fig. 4A and C). Both Bipy and o-Phe are inhibitors of Fenton-type reactions known to block the formation of HO. from H2O2.15 Taken together, a HO•-generating reaction via iron-catalyzed H2O2 decomposition (i.e., Fenton-type reaction) is a likely path for O3-dependent damaging of the cells.
Some ROS scavengers applied to the cells were shown to be toxic by themselves by enhancing the cell death in the absence of O3, thus their impacts on the O3 toxicity could not be evaluated. Such toxic ROS scavengers include copper salicylate (an SOD mimic, highly toxic at 0.1 mM) and N-acetylcysteine (radical scavenger, highly toxic at 0.1 mM). Tiron (scavenger of O2•-) showed no inhibitory effects over the range from 1 to 50 mM (toxic at 50 mM) and the O3-induced cell death was somehow enhanced at 1 mM. Other chemicals namely diphenyleneiodonium chloride (NADPH oxidase inhibitor, 50–300 mM), Cu,Zn-SOD from bovine erythrocytes (300 units/ml), and Fe-EDTA (an SOD mimic, 0.2–1.0 mM) showed no effects on the toxicity of O3.
Effect of Ca2+ chelators and a Ca2+ ionophore.
As shown in Figure 4B and D, addition of 5 mM BAPTA, a membrane-impermeable Ca2+ chelator active at physiological range of pH, resulted in significant inhibition of the O3-induced cell death in both cell lines. Similarly, addition of 10 mM EGTA, a chelator of Ca2+, known to be less active at physiological range of pH, resulted in inhibition of the O3-induced cell death to a lesser extent (data not shown). These results suggested that the uptake of extracellular Ca2+ is required for active cell death induction.
In addition, we tested the effect of a Ca2+ ionophore, A23187 (10 mM) on the O3-induced cell death in Bel-B cells (Fig. 4F). Pretreatment with A23187 significantly enhanced the O3-induced cell death. A23187-dependent enhancement in O3-induced cell death was also observed in Bel-W3 cells (data not shown).
Thiols as prooxidants.
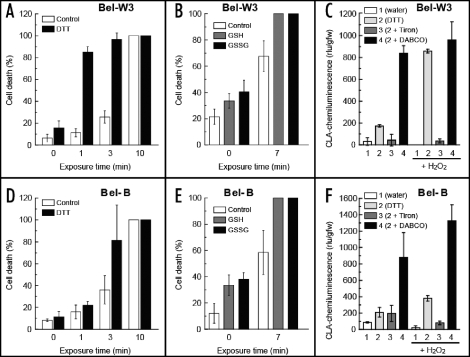
Behaviors of some thiols (expected to act as antioxidants) were very much confusing (Fig. 5). Pretreatment of tobacco cells with 10 mM dithiothreitol (DTT) resulted in drastic enhancement of O3-induced cell death. While 1 min of treatment with ozonized air was not sufficient for inducing the cell death in both cell lines, such short exposure to O3 killed nearly 90% of the DTT-pretreated Bel-W3 cells (Fig. 5A). Such synergic effect of O3 and DTT on increased cell death was observed also in Bel-B cells after being exposed to ozonized air for 3 min (cell death was doubled, Fig. 5D).
Figure 5.
Actions of thiols as prooxidants synergistically enhancing the O3-induced cell death in tobacco cell suspension culture. Tobacco cells (Bel-W3 cells, A; Bel-B cells, D) were concurrently treated with 10 mM dithiothreitol (DTT) and O3. After 0, 1, 3 or 10 min of exposure to O3, the cells were incubated for 4 h to allow active cell death. Then the cells were stained with Evans blue for 1 h. Similarly GSH and GSSG (0.5 mM) were cotreated with O3 and cell death was counted (B and E). Effects of DTT (0.1 mM) as a prooxidant generating and/or enhancing the generation of O2- in the presence and absence of 0.1 mM H2O2, 1 mM Tiron, and 1 mM DABCO in the cultures. 1, water control; 2, DTT alone; 3, DTT + Tiron; 4, DTT + DABCO, horizontal bars, plus H2O2 (0.5 mM); rlu, relative luminescence units. Four randomly chosen digital images of different portion of each sample under microscopes were acquired and stained cells were counted (A, B, D and E). Thus, each data point reflects the mean and S.E. (vertical bars, n = 4). Typical data from three replications are shown.
Interestingly, additions of glutathione at both reduced (GSH, 0.5 mM) and oxidized (GSSG, 0.5 mM) states resulted in enhancement of cell death (Fig. 5B and E). Since both GSH and GSSG similarly enhanced the O3-induced cell death, we understood that both forms likely act as prooxidants in the presence of O3. These phenomena are indicative of the complicated chemistry of thyl radicals reported for toxicity of variety of thiols in which unpaired electrons (radicals) are produced and relayed between RSH and RSSR, and finally conveyed to molecular oxygen to yield O2•-.17 In support of such possible thiol-dependent events, we observed that addition of 0.1 mM DTT to Bel-W3 and Bel-B cells resulted in generation of O2•- monitored with chemiluminescence of CLA (Fig. 5C and F). The observed chemiluminescence of CLA was sensitive to 1 mM Tiron but not to 1 mM DABCO (somehow enhanced). In combination with H2O2, DTT induced much greater generation of O2•- especially in Bel-W3 cells (Fig. 5C). In addition, thiol-H2O2 synergetic action reportedly resulting in inactivation of ROS-detoxifying enzymes such as ascorbate peroxidase18 may be a likely cause of thiol-enhanced O3 toxicity to be examined in the future experiments. Since glutathione at both redox states behaved as prooxidants here, GSH recycling enzymes such as glutathione reductase may not play a key role in protection of plants from O3.
Cell death proceeds after the acute O3 exposure.
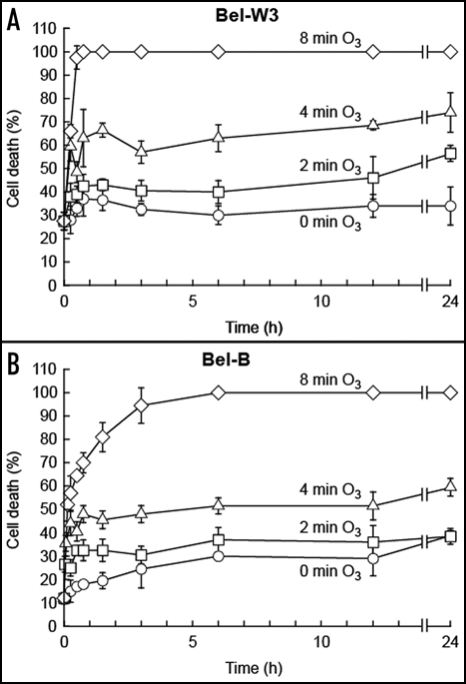
The cells were treated with ozonized air for 0 (control), 2, 4 or 8 min and further incubated in the absence of O3. By counting from the time zero, cells were sampled at 0, 0.25, 0.5, 0.75, 1.5, 3, 6, 12 and 24 h and stained for 1 h with Evans blue. As shown in Figure 6, increases in the rate of cell death can be expressed as the functions of both the time of O3 exposure and the incubation time after the pulse of O3. It became clear that the number of dead cells increase with time. Especially the increases in cell death after (not during) the O3 exposure was impressive in both cell lines, thus indicating that the pulse of O3 initiates the series of reaction required for development of cell death and the actual cell killing process can proceed during the post-ozone incubation in the O3-free air. The time lags for development of cell death after the O3 pulses were much smaller in Bel-W3 cells compared with Bel-B cells. Time-dependent increases in dead cells were also observed at the relatively late stage between 12 and 24 h after the O3 pulse.
Figure 6.
Time-dependent development of O3-induced cell death in Bel-W3 and Bel-B tobacco cells. Cell death in Bel-W3 cells (A) and Bel-B cells (B) were induced by exposing the 10 ml of cell suspensions to the pulse of ozonized air lasting for 0, 2, 4, and 8 min. O3-treated and pretreated cell suspensions (0.2 ml) were sampled and transferred to a 1.5 ml tube at the end of O3 exposure. Cells were totally incubated for up to 24 h (O3 exposure plus following incubation total the time indicated up to 24 h in each treatment). After occasional sampling as indicated in the figure (at 0, 0.25, 0.5, 0.75, 1.5, 3, 6, 12, 24 h), cells were stained with Evans blue for 1h. Three randomly chosen digital images of different portion of each sample under microscopes were acquired and stained cells were counted. Thus, each data point reflects the mean and S.E. (vertical bars, n = 3). Typical data from triplicate experiments. As controls, 10 ml of Bel-W3 cells and Bel-B cells were exposed to the pulse of O3-free air for 0, 2, 4, and 8 min and incubated for 24 h. The levels of cell death measured after 24 h of static incubation were within 15–22% in Bel-W3 cells, and 24.5-28% in Bel-B cells.
To test the hypothesis that oxidative cell death (mediated by O3-induced ROS) proceeds after the pulse of O3 treatment, the effects of post-ozone applications of three strong inhibitors of cell death related to the formation of HO., namely Bipy, o-Phe and DMTU, were examined (Fig. 7A and D). Development of O3-induced cell death in Bel-W3 cells during 2 h of post-ozone incubation was effectively blocked by post-ozone addition of Bipy, o-Phe and DMTU. These data confirmed the involvement of HO. in the post-ozone cell death development in tobacco cells.
Figure 7.
Effects of post-ozone application of ROS scavengers and metal chelators on O3-induced cell death in Bel-W3 and Bel-B tobacco cells. Cell death of Bel-W3 cells (A–C) and Bel-B cells (D–F) were induced by exposing the cells to ozonized air (for 0, 7, 15 min). Immediately after the exposures to O3, HO.-related inhibitors (A, D), namely Bipy (2 mM), o-Phe (2 mM) and DMTU (50 mM); two upstream ROS scavengers (B, E), namely catalase (2000 units/ml) and DABCO (10 mM); and a Ca2+-chelator (C,F), BAPTA (5 mM) were added to the cell suspensions. For development of cell death, cells were incubated for further 2 h prior to cell death staining with Evans blue. Four randomly chosen digital images of different portion of each sample under microscopes were acquired and stained cells were counted. Thus, each data point reflects the mean and S.E. (vertical bars, n=4). Typical data from triplicates are shown.
Probably because the time points around 6–8 min are critical for determining the outcome of O3 exposure in Bel-B cells, only 1 min longer exposure to O3 often results in twice greater increase in cell death. In Bel-B cells treated with 8 min-long pulse of O3, the protective effect of DMTU, Bipy and o-Phe could be confirmed (data not shown).
Post-ozone application of catalase (2000 units/ml) also rescued the O3-treated cells (Fig. 7B and E), confirming that H2O2 is involved in the O3-induced cell death development. Effect of catalase supplementation was more prominent in Bel-B cells compared to Bel-W3 cells. Assuming that H2O2 is a key factor inducing the cell death, total catalase activity likely determines the fate of the O3-treated cells, thus the survival of the catalase-supplemented cells can be expressed as follows:13 C > D, C = B + A; where D is the death inducing level of H2O2 produced, C is the capacity of catalase, B is the basal catalase activity, and A is the additional (supplemented) catalase activity. Above formulae indicate that Bel-B culture should have greater ‘C’ value compared with Bel-W3 culture. As the same amounts of ‘A’ was added to both culture, the difference in ‘C’ value is due to the difference in ‘B’ value which is the endogenous catalase level. In the below section, basal catalase activities in both cell lines were compared.
In contrast to H2O2- and HO.-related chemicals, post-ozone applications of 10 mM DABCO (Figs. 7B and E) and 5 mM BAPTA (Figs. 7C,F) showed no protective effect. Therefore, it is conclusive that 1O2 and Ca2+ participate only in the early cell death initiating events during O3 exposure but not during the post-ozone cell death development.
Catalase activity.
Since supplementation of catalase to Bel-W3 cells significantly lowered the rate of cell death induced by 7 min-exposure to ozonized air while much of Bel-B cells survived at the same condition without supplementation of catalase (Fig. 4E), we assumed that Bel-B cells may be richer in endogenous catalase activity compared to Bel-W3 cells. Here, in vivo catalase assay was carried out for two tobacco cell lines, by monitoring the H2O2-dependent O2 evolution using an oxygen electrode (Fig. 8). Since monitoring of catalase activity based on H2O2 consumption is not appropriate for in vivo systems due to the presence of other H2O2-consuming enzymes such as peroxidase, the use of oxygen electrode is more reliable as discussed elsewhere.19
Figure 8.
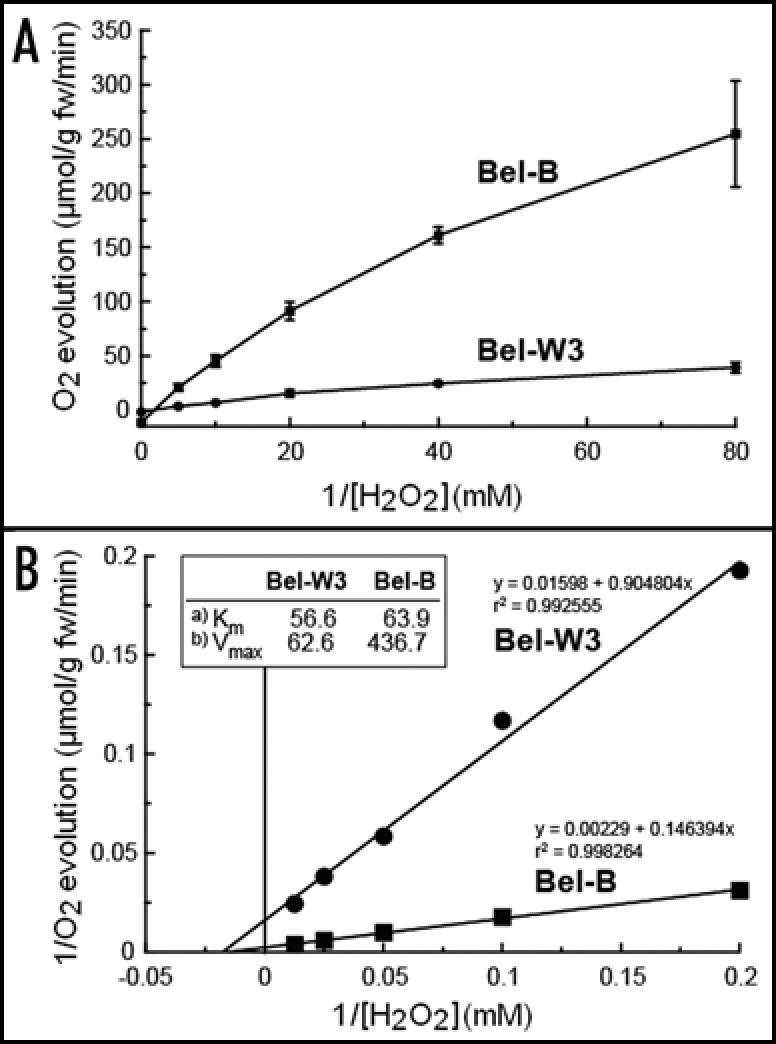
In vivo catalase activities in Bel-W3 and Bel-B tobacco cell suspension cultures. (A) Oxygen evolved after addition of the indicated concentrations of H2O2 to 3 ml cell culture (three day-old cells), was monitored with an oxygen electrode. (B) Lineweaver-Burk kinetic analysis. Inset, apparent Km values for H2O2 (A) and Vmax values for O2 evolution (µmol/g fw/min) (B) in two cultures were compared. Typical data from five repeated experiments.
The H2O2-dependent evolution of O2 was superior in Bel-B cells compared to Bel-W3 cells in the range of H2O2 concentrations examined (Fig. 8A). The Vmax values for Bel-W3 and Bel-B cells determined by graphical analysis of the data with Lineweaver-Burk kinetic analysis were 62.6 and 436.7 µmol O2/g fw/min, respectively (Fig. 8B). The apparent Km values for H2O2 in Bel-B and Bel-W3 were 56.6 and 63.9 mM, respectively. These data suggest that Bel-B cells scavenge H2O2 by bulky catalase (higher Vmax) with maxsimilar affinity for H2O2 (similar Km), and this may be reflecting the tolerance of Bel-B cells to O3-induced ROS in part, although involvements of other endogenous scavengers (it can be non-enzymatic) acting against HO. may be of great importance too.
Detection of O3-induced ROS.
Since above experiments were indicative of the involvement of ROS in acute O3 toxicity, O3-induced generations of ROS were monitored with chemiluminescence (Fig. 9A). Unexpectedly, there was a strong background chemiluminescence detectable during O3 exposure without addition of any chemiluminescent probe to the cells (Fig. 9A, bottom). Probably, such background light emission was due to the reactions between O3 and some cellular components.
Figure 9.
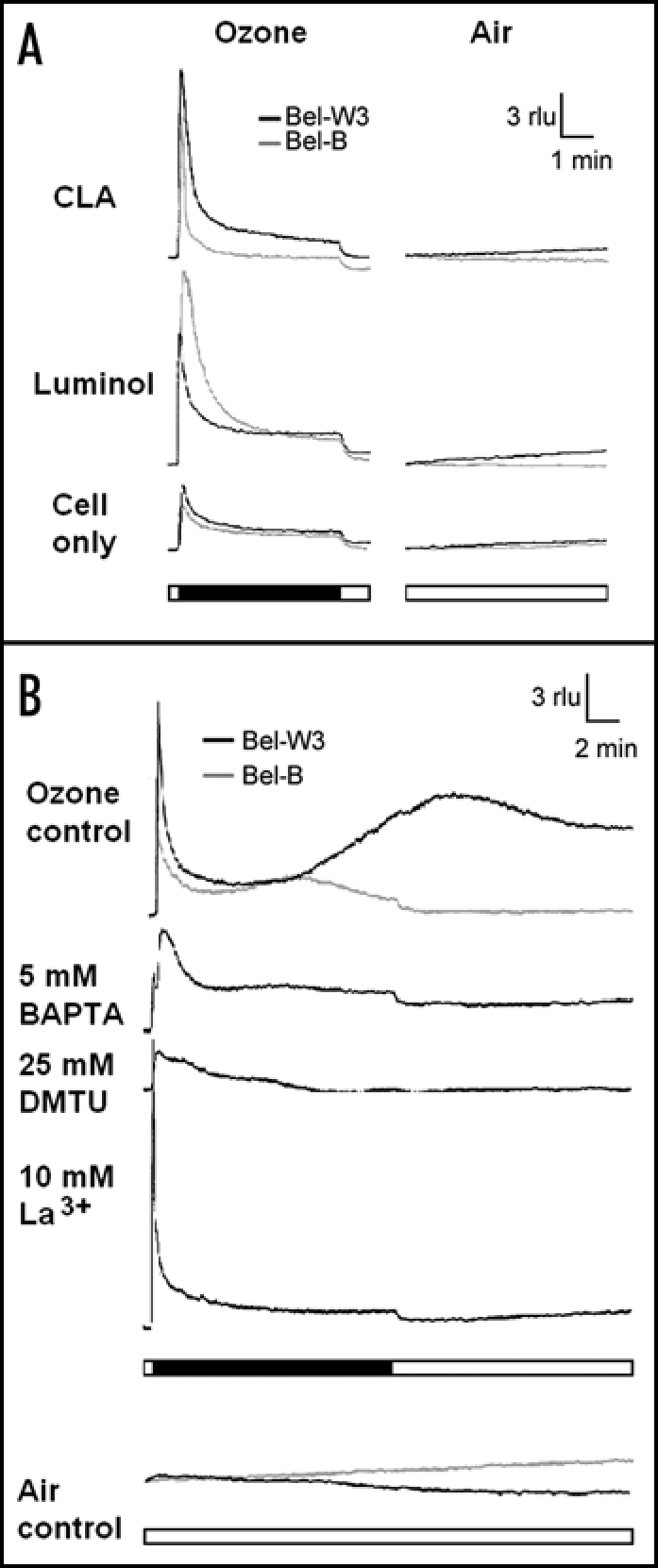
Luminescence analyses for monitoring of ROS production and changes in [Ca2+]c in O3-exposed Bel-W3 and Bel-B tobacco cell suspension cultures. (A) Detection of ROS with chemiluminescent probes. Typical patters of luminol-dependent (top) and CLA-dependent (middle) chemiluminescence spikes induced by O3 exposure. Intrinsic high background chemiluminescence in the absence of chemiluminescence probes emitted by cell cultures exposed to (bottom). (B) O3-induced changes in [Ca2+]c monitored with aequorin luminescence. rlu, relative luminescence units. Horizontal bars (closed), length of O3 exposure (5 min for ROS detection and 15 min for [Ca2+]c monitoring). Horizontal open bars, length of control air blowing. Data in (A and B) were obtained with wild type cell lines and transgenic cell lines expressing aequorin, respectively. Typical data from 5–6 times repeated experiments.
In the presence of luminol, yields of chemiluminescence were largely enhanced in both cultures exposed to O3, thus indicating the presence of O3-induced H2O2 (Fig. 9A, top). Addition of CLA which reports the presence of both O2•- and 1O2 (to a lesser extent) also resulted in significant enhancements of the light yields (Fig. 9A, middle). Since CLA is responsive to both O2•- and 1O2, Tiron and DABCO can be used for determination of the species involved.20 In the presence of 5 mM DABCO, the CLA-chemiluminescence was inhibited by 47% and 63% in Bel-W3 and Bel-B cells, respectively. Instead, Tiron at 5 mM somehow enhanced the CLA-dependent yield of light by 364% and 24% in Bel-W3 and Bel-B cells, respectively. Since Tiron showed no inhibitory effect, the observed chemiluminescence in the presence of CLA may not be reflecting the presence of O2•-, while the production of 1O2 was supported by the action of DABCO.
The O3-induced ROS production in cell suspensions were also confirmed using two fluorescent probes for ROS, namely hydroxyphenyl fluorescein and aminophenyl fluorescein. Since hydroxyphenyl fluorescein and aminophenyl fluorescein are probes originally designed for detection of HO• in biological systems, the increases in hydroxyphenyl fluorescein- and aminophenyl fluorescein-dependent fluorescence in the O3-exposed cell suspension supported our view that HO. is produced from O3 (data not shown). However, further confirmation of HO production in these tobacco cells by other means such as ESR, are required in the future experiments.
Monitoring of cytosolic Ca2+ concentration ([Ca2+]c).
The changes in [Ca2+]c was monitored with aequorin luminescence using two sets of transgenic cell lines expressing aequorin, derived from Bel-B and Bel-W3 cells (Fig 9B). As implicated by the action of Ca2+ chelators, we obtained the evidence for the increases in [Ca2+]c in response to the pulse of O3 in the cells we used. Spikes of the chemiluminescence observed in the absence of luminophore, coelenterazine may be due to nonspecific background light emitting reactions as observed with wild type cells exposed to O3 (Fig. 9A). In Bel-W3 cells incubated with 1 µM coelenterazine for over 8 h, the observed changes in aequorin luminescence were biphasic events consisting of an immediate spike (nonspecific) and a gradual increase (reflecting the [Ca2+]c increase) attaining the peak level at around 10 min after initiation of the O3 exposure (Fig 9B, top). We observed that a Ca2+ chelator, BAPTA (5 mM) and a Ca2+ channel blocker, La3+ (10 mM) completely inhibited the induction of [Ca2+]c increases by O3, indicating the influx of extracellular Ca2+ into the cells via activation of Ca2+ channels. DMTU, a scavenger of HO• (25 mM) also inhibited the increases in [Ca2+]c suggesting that O3-induced ROS triggers the Ca2+ influx. Also in Bel-B cell line expressing aequorin, we observed the biphasic nature of Ca2+ influx consisting of the immediate spike and the slow phase of [Ca2+]c increase (Fig 9B). However, the secondary phase of [Ca2+]c increase in Bel-B cells was shown to be much lower compared to the Bel-W3 cells. The fact that O3-tolerant cells are less responsive to O3 in induction of Ca2+ influx, is consistent with other observations supporting the view that sufficient Ca2+ influx is required for O3-mediated acute cell death. Assuming that Ca2+ plays a role as a signaling molecule mediating the cell death mechanism, there may be some relationships between the level of [Ca2+]c changes and extent of the cell death in the O3-exposed cells. Thus, this may be another key step in addition to the production of ROS, determining the outcome of the O3 actions in tobacco cells.
The biphasic patterns of luminescence yield in aequorin-expressing Bel-W3 cells exposed to O3 pulse are very similar to the changes in luminescence yield in O3-fumigated Arabidopsis thaliana seedlings expressing aequorin.21 Especially, the response of the aerial part of the seedlings exposed to sub-ppm level of O3, were very similar to the Bel-W3 cells' O3-induced calcium response.
Lipid peroxidation.
O3 likely damages the cellular membranes and such damaging impacts of O3 often associate with concomitant lipid peroxidation. Here, we observed the increase in TBARS as the measure of O3-induced lipid peroxidation, and found that TBARS attains the detectable levels only after long exposure (0.5–1 h) to O3 (data not shown), and thus, concluded that lipid peroxidation is a consequence or concomitant process, but not the cause of O3-induced acute cell death.
Involvement of hormones?
It has been suggested that many of biological actions of O3 in planta are mediated by stimulated productions of ethylene and salicylic acid.22–25 We examined the impacts of ethylene and salicylic acid on the induced cell death (Fig. 10). Incubation of the cell cultures with 100 ppm gaseous ethylene sealed in a 50-ml tube with a silicone injection port (with 40 ml gas phase and 10 ml cell suspension) on a gyratory shaker for 10 min prior to O3 treatment resulted in no change in the O3-induced cell death. In addition, ethylene added alone induced no cell death. Similarly, the effect of norbornadiene (inhibitor of ethylene action) vaporized for 30 min in a gas-tight tube (1.2 µl liquid in 40 ml gas phase above 10 ml culture) was tested and no impact was observed (data not shown).
Figure 10.

Effects of ethylene and salicylate on O3-induced cell death in tobacco cell cultures. Effects of two phytohormones, ethylene (C2H4, 100 ppm, 10 min prior to the O3 pulses) and sodium salicylate (NaSA, 1 mM) in Bel-W3 (A) and Bel-B (B) cells. Ethylene pretreatment was carried out by injecting the gaseous ethylene into 50-ml gas-tight tube with a silicone rubber port (containing 10 ml of cell suspension) through the needle of a syringe. The cells were incubated with 100 ppm ethylene for 10 min with continuous shaking. Sodium salicylate was simply added prior to the O3 pulse. Then Bel-W3 and Bel-B cells were exposed to ozonized air for 0, 7, 15 min. For development of cell death, cells were incubated for further 2 h after O3 exposure. Four randomly chosen digital images of different portion of each sample under microscopes were acquired and stained cells were counted. Thus, each data point reflects the mean and S.E. (vertical bars, n = 4). Typical data from duplicate experiments are shown.
Although enhancement of the O3 (7 min pulse)-induced cell death by 1 mM sodium salicylate was observed, treatment with sodium salicylate (added alone) induced no detectable cell death in both cell lines (Fig. 10A). Salicylate is well known as a natural and strong inhibitor of catalase of various origins, and at 1 mM the in vivo catalase activity in tobacco cell suspension culture can be completely inhibited.13 Therefore the enhanced cell death in the salicylate-pretreated cells could be attributed to the lowered catalase activity. This view is consistent with other observations that the cell culture richer in catalase was tolerant to O3 (Fig. 8) and addition of exogenous catalase conferred the O3 tolerance to the catalase-poor O3-sensitive cell culture (Fig. 4E). Since extremely high concentration of salicylate (1 mM) and ethylene (100 ppm), when treated alone, failed to induce the cell death within 2 h of incubation, it is hard to conclude that salicylate or ethylene is the mediator of the O3-induced acute cell death. These results suggest that the roles for ethylene and salicylate in the O3-dependent acute cell death induction are very much limited.
Discussion
O3 concentrations and exposure time.
Acute exposure to high O3 concentrations can induce chlorosis and necrotic lesion whereas accelerated leaf senescence can be observed in case of chronic exposure to low O3 levels in leaves of O3-sensitive plants derived from various plant species including potato,26 Arabidopsis thaliana25 and tobacco.8 Through our preliminary works, we have confirmed that exposure of Bel-W3 plants and leaves to acute single pulse of high O3 concentration often results in induction of the symptoms reportedly inducible by long-term chronic exposure to low O3 concentrations, indicating that O3 perception mechanisms sense the cumulative effect of O3 concentration and the length of exposure. For example, Bel-W3 tobacco plants exposed to 90–135 ppb O3 for 20 consecutive days (O3 applied for 8 h a day) reportedly display necrotic and chlorotic spots on leaves, acceleration of leaf senescence, and chlorophyll degradation with greater loss of chlorophyll a over chlorophyll b.27 All of such chronic O3-induced symptoms can be reproduced by very shortly exposing the Bel-W3 plants or leaf discs to high concentrations of O3 (10–20 ppm; 1–4 h for spotting, 4–8 h for senescence and chlorophyll degradation), if the conditions (such as the post-ozone incubation at least for 12–24 h, and etc.) were precisely controlled (Hirono et al., unpublished results). Assuming that the formation of lesions on Bel-W3 leaves is initiated by certain countable measure of O3 exposure effect, we can understand that two different conditions leading to induction of lesion on Bel-W3 leaves, namely the long-term exposure to low O3 concentrations and short-term exposure to high O3 concentrations, are roughly equivalent in some limited ranges of time and concentrations. Thus, we can simply score the exposure effect with 90–135 ppb (ca. 0.1 ppm) O3 for 20 days (but 8 h a day only) as to 16 ppm.h, and the acute O3 exposure effect with 10–20 ppm O3 for 1 h can be scored as 10–20 ppm.h. Although we are not certain if it is merely a coincidence that similar ranges of O3 exposing scores (16 and 10–20 ppm.h) likely result in formation of lesions on Bel-W3 leaves, we would like to propose a conjecture that such simple calculation helps roughly estimating the impacts of O3 to plants.
There is some advantage to shorten the time required for observing the plants' responses to O3. Apparently, ecological time scales spanning for many days does not fit for biochemical and cell-biological research purposes. Since secondary effects of senescence or many of other secondary factors involved in photosynthesis, growth, nutritional allocations, metabolisms and so on, possibly affect the outcome of O3 exposure in plants, the plants' responses should be evaluated within short periods in order to see more direct early impacts of applied O3. Therefore, the present study employed the short pulses (1–15 min) of highly ozonized air.
Protective roles of scavengers in animal systems.
In animal systems, biochemical and cell biological impacts of O3 inhalation have been well documented. Following exposure to O3, a drastic increase in cell permeability can be induced in the mammalian airway and as a consequence, fluxes of macromolecules such as that of albumin across the membrane occurs.28 Such damaging events may be mediated with production of ROS derived from O3 since ROS could be detected in the airway tissues of animals exposed to O3. In guinea pig, in vivo exposure to O3 results in intracellular generation of ROS (monitored with a fluorescent dye) and cellular damages being associated with lipid peroxidation in the airway epithelium.29 The detected changes in intracellular fluorescence in O3-exposed airway tissues were shown to be sensitive to some scavengers of HO. such as sodium formate. Early studies with rat lungs documented the effects of ROS scavengers against the O3 toxicity. While lipid soluble scavengers such as β-carotene and α-tocopherol showed no protective effect, water soluble scavengers especially DMTU, a HO• scavenger, effectively protected the lungs from O3-induced damages.30,31 In support of above view, recent study has demonstrated the detection of O3-induced formation of HO. (measured as the hydroxylation of an aromatic probe) in the rat lung.32 Other studies further confirmed the lung protective effect of DMTU in O3-treaded rats.28 These studies strongly suggested that O3-induced HO• plays a key role in the O3-induced cell damages and the use of DMTU is a possible choice to protect the living cells.
Although our study could not show the sites of actions of O3 and ROS, we are thinking that penetration of O3 into the cells are not necessary for inducing the oxidative cell damages by analogy to the model for hemolysis-dependent propagation of oxidative cell death in fish erythrocytes exposed to O3, in which hemoglobin or heme iron (which behave as Fenton-type catalysts depending on the oxidation status) released by hemolysis participates in the O3-dependent formation of H2O2 and lipid peroxidation attacking other cells from the extracellular space.33 Since H2O2 is permeable to plant cell membrane,19 extracellularly produced H2O2 may readily penetrate through the plasma membrane and may contribute to damages to the intracellular components.
Chemistry of O3-ROS conversions in aqueous phase.
At alkaline conditions, O3 readily reacts with OH- to yield ozonide (O3-) and HO, then resultant O3- can be further degraded by reaction with water yielding O2•-, O2 and OH-34 as summarized below:
| [1] |
| [2] |
Although O3-dependent formations of HO• and O2•- are not likely to take place at high paces in the aqueous phase at physiological pHs,35 some solutes such as phenolics35 or high concentration of Cl-36 catalyzes the O3-dependent formation of HO•, indicating that HO• can be generated non-enzymatically even at the physiological ranges of pH.
Similarly, a model of O3-dependent damages to biological systems (around the gas/liquid interfaces in the respiratory organs in animals) involving 1O2 and/or O3- was recently proposed, in which the actions of O3 leading to the oxidation of cellular components such as plasma membrane, is mediated by 1O2 and/or O3- derived from O3 after reacting with extracellular ascorbate or thiols.37 Ascorbate-dependent generation of 1O2 during exposure to O3 has been demonstrated also in plant leaves,38 and some other studies suggested that generation of 1O2 from O3 occurs in the presence of variety of biomolecules such as ascorbate, NADPH, NADH, S-containing amino acids and peptides (methionine, cysteine and reduced glutathione), and some proteins such as albumin as the active catalysts.39,40
The fate of 1O2 has been well studied in the dye-mediated photosensitization events known as photodynamic actions. According to ESR studies, conversions of photochemically produced 1O2 into H2O2 and HO• occur in the presence of biological molecules such as NADPH as catalysts, without the involvement of O2•-.41 However, in such experiments, H2O2 and HO. were shown to be produced via paralleled independent paths since catalase showed no inhibitory effect against the yield of HO•. Contrary, in case of cell death induction in tobacco cells tested here, both catalase and HO•-related chemicals effectively lowered the level of cell death (Fig. 4A and C) suggesting that H2O2 takes place in the HO•-mediated action. Therefore, it is natural to conclude that H2O2 is a major source of HO. via Fenton-type reactions in O3-exposed tobacco cells.
Likely paths of O3 actions leading to acute cell death.
Lastly, we would like to discuss the possible oxidative and signaling pathways for induction of cell death by O3 (Fig. 11). One of the key findings in the present study was that the oxidative reactions leading to cell death can be dissected into (1) early O3-dependent reactions and (2) post-ozone production (or internalization) of ROS (Fig. 11A). Our present experiments with various inhibitors (ROS scavengers and metal chelators) suggested that the O3-induced acute cell death is mediated by ROS members generated by the path involving 1O2, H2O2 and HO• (Fig. 11B). Since two chelators of Fe2+ completely inhibited the O3-induced cell death, the pathway directly generating the HO• from O3 (and HO-) was denied, and instead possible involvement of Fenton-type reaction catalyzed by Fe or Cu in which H2O2 can be degraded into the lethal level of HO. was suggested. Due to its high reactivity, HO may react within short diffusion distance with any organic molecule and thus it is unlikely that HO• serve as a migrating ROS or a signaling molecule. Instead, we should better understand that HO. acts at the site of generation and thus HO• is the final form of oxidative relay attacking the cellular components. HO• released from H2O2, may appear twice in the mechanism, firstly for penetration of membrane or stimulation of ion channels allowing the movements of solutes including Ca2+, and secondly after H2O2 was taken up by the cells (H2O2 is the only ROS stably permeable to the membrane) or endogenously produced.
Figure 11.
Possible paths for O3-dependent generation of ROS leading to cell death in tobacco cells. There are two likely paths starting from O3. One pathway is a pH-dependent and purely consisting of radical reactions. This pathway is dominant in alkaline conditions since O3 can readily react with bulky OH- to yield HO. and O3-. The other likely path starting from O3 proceeds at physiological pH, via unknown mechanism involving 1O2, H2O2 and HO.. The pathways connecting the experimentally suggested components are shown with solid arrows. Other paths lacking evidence were shown with broken arrows.
Calcium signaling initiated by O3-dependent Ca2+ influx possibly links the primary and secondary oxidative bursts. Although NADPH oxidase often appears as a candidate enzyme system for oxidative burst, we have to consider alternative ROS-generating mechanism at down-stream of Ca2+ since diphenyleneiodonium chloride, an NADPH oxidase inhibitor, failed to inhibit the cell death.
Acknowledgements
We acknowledge Prof. A.J. Trewavas for permitting the use of aequorin-expressing plant materials, and Prof. M. Isobe and Dr. M. Kuse for synthesizing coelenterazine. Part of digital microscopic analysis was carried out at the Instrumentation Center, The University of Kitakyushu. T. Kawano was supported by Grant-in-Aid (No. 18780047) from Ministry of Education, Culture, Sports, Science and Technology.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=3518
References
- 1.Ashmore MR. Assessing the future global impacts of ozone on vegetation. Plant Cell Environ. 2005;28:949–964. [Google Scholar]
- 2.Fiscus EL, Booker FL, Burkey KO. Crop responses to ozone: Uptake, modes of action, carbon assimilation and partitioning. Plant Cell Environ. 2005;28:997–1011. [Google Scholar]
- 3.Baier M, Kandlbinder A, Golldack D, Diez KL. Oxidative stress and ozone: Perception, signalling and response. Plant Cell Environ. 2005;28:1012–1020. [Google Scholar]
- 4.Mehlhorn H, Tabner BJ, Wellburn AR. Electron spin resonance evidence for the formation of free radicals in plants exposed to ozone. Physiol Plant. 1990;79:377–383. [Google Scholar]
- 5.Runeckles VC, Vaartnou M. EPR evidence for superoxide anion formation in leaves during exposure to low levels of ozone. Plant Cell Environ. 1997;20:306–314. [Google Scholar]
- 6.Overmyer K, Brosche M, Pellinen R, Kuittinen T, Tuominen H, Ahlfors R, Keinaenen M, Saarma M, Scheel D, Kangasjaervi J. Ozone-induced programmed cell death in the Arabidopsis radical-induced cell death 1 mutant. Plant Physiol. 2005;137:1092–1104. doi: 10.1104/pp.104.055681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schraudner M, Moeder W, Wiese C, Van Camp W, Inze D, Langebartels C, Sandermann H. Ozone-induced oxidative burst in the ozone biomonitor plant, tobacco Bel W3. Plant J. 1998;16:235–245. doi: 10.1046/j.1365-313x.1998.00294.x. [DOI] [PubMed] [Google Scholar]
- 8.Heggestad HE. Origin of Bel-W3, Bel-C and Bel-B tobacco varieties and their use as indicators of ozone. Environ Polllut. 1991;74:264–291. doi: 10.1016/0269-7491(91)90076-9. [DOI] [PubMed] [Google Scholar]
- 9.DeAlvare LR, Goda K, Kimura T. Mechanism of superoxide anion scavenging reaction by bis-(salicylato)-copper (II) complex. Biochem Biophys Res Commun. 1976;69:687–694. doi: 10.1016/0006-291x(76)90930-x. [DOI] [PubMed] [Google Scholar]
- 10.Kawano T, Adachi M, Kurata H, Azuma R, Shimokawa K. Calcium-dependent catabolism of phaeophorbide a in tomato fruit. J Japan Soc Hort Sci. 1999;68:810–816. [Google Scholar]
- 11.An G. High efficiency transformation of cultured cells. Plant Physiol. 1985;79:568–570. doi: 10.1104/pp.79.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi K, Isobe M, Knight MR, Trewavas AJ, Muto S. Hypoosmotic shock induces increase in cytosolic Ca2+ in tobacco suspension-culture cells. Plant Physiol. 1997;113:587–594. doi: 10.1104/pp.113.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawano T, Sahashi N, Takahashi K, Uozumi N, Muto S. Salicylic acid induces extracellular superoxide generation followed by an increase in cytosolic calcium ion in tobacco suspension culture: The earliest events in salicylic acid signal transduction. Plant Cell Physiol. 1998;39:721–730. [Google Scholar]
- 14.Nakano M, Sugioka K, Ushijima Y, Goto T. Chemiluminescence probe with Cypridina luciferin analog, 2-methyl-6-phenyl-3,7-dihydroimidazo [1,2-a] pyrazin-3-one, for estimating the ability of human granulocytes to generate O2- Anal Biochem. 1986;159:363–369. doi: 10.1016/0003-2697(86)90354-4. [DOI] [PubMed] [Google Scholar]
- 15.Kawano T, Muto S. Mechanism of peroxidase actions for salicylic acid-induced generation of active oxygen species and an increase in cytosolic calcium in tobacco suspension culture. J Exper Bot. 2000;51:685–693. [PubMed] [Google Scholar]
- 16.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 17.Wlodek L. Beneficial and harmful effects of thiols. Pol J Pharmacol. 2002;54:215–223. [PubMed] [Google Scholar]
- 18.Chen GX, Asada K. Inactivation of ascorbate peroxidase by thiols requires hydrogen peroxide. Plant Cell Physiol. 1992;33:117–123. [Google Scholar]
- 19.Kawano T, Pinontoan R, Uozumi N, Miyake C, Asada K, Kolattukudy PE, Muto S. Aromatic monoamine-induced immediate oxidative burst leading to an increase in cytosolic Ca2+ concentration in tobacco suspension culture. Plant Cell Physiol. 2000;41:1251–1258. doi: 10.1093/pcp/pcd052. [DOI] [PubMed] [Google Scholar]
- 20.Yokawa K, Suzuki N, Kawano T. Ethanol-enhanced singlet oxygen-dependent chemiluminescence interferes with the monitoring of biochemical superoxide generation with a chemiluminescence probe, Cypridina luciferin analog. ITE Lett Batter New Technol Medic. 2004;5:49–52. [Google Scholar]
- 21.Evans NH, McAinsh MR, Hetherington AM, Knight MR. ROS perception in Arabidopsis thaliana: The ozone-induced calcium response. Plant J. 2005;41:616–626. doi: 10.1111/j.1365-313X.2004.02325.x. [DOI] [PubMed] [Google Scholar]
- 22.Tamaoki M, Nakajima N, Kubo A, Aono M, Matsuyama T, Saji H. Transcriptome analysis of O3-exposed Arabidopsis reveals that multiple signal pathways act mutually antagonistically to induce gene expression. Plant Mol Biol. 2003;53:443–456. doi: 10.1023/B:PLAN.0000019064.55734.52. [DOI] [PubMed] [Google Scholar]
- 23.Vahala J, Ruonala R, Keinanen M, Tuominen H, Kangasjarvi J. Ethylene insensitivity modulates ozone-induced cell death in birch. Plant Physiol. 2003;132:185–195. doi: 10.1104/pp.102.018887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogawa D, Nakajima N, Sano T, Tamaoki M, Aono M, Kubo A, Kanna M, Ioki M, Kamada H, Saji H. Salicylic acid accumulation under O3 exposure is regulated by ethylene in tobacco plants. Plant Cell Physiol. 2005;46:1062–1072. doi: 10.1093/pcp/pci118. [DOI] [PubMed] [Google Scholar]
- 25.Yaeno T, Saito B, Katsuki T, Iba K. Ozone-induced expression of the Arabidopsis FAD7 gene requires salicylic acid, but not NPR1 and SID2. Plant Cell Physiol. 2006;47:355–362. doi: 10.1093/pcp/pci253. [DOI] [PubMed] [Google Scholar]
- 26.Saitanis CJ, Riga-Karandinos AN, Karandinos MG. Effects of ozone on chlorophyll and quantum yield of tobacco (Nicotiana tabacum L.) varieties. Chemosphere. 2001;42:945–953. doi: 10.1016/s0045-6535(00)00158-2. [DOI] [PubMed] [Google Scholar]
- 27.Bhalla DK. Alteration of ozone-induced airway permeability by oxygen metabolites and antioxidants. Toxicol Lett. 1994;73:91–101. doi: 10.1016/0378-4274(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 28.Chen LC, Qu Q. Formation of intracellular free radicals in guinea pig airway epithelium during exposure to ozone. Toxicol Appl Pharmacol. 1997;143:96–101. doi: 10.1006/taap.1996.8073. [DOI] [PubMed] [Google Scholar]
- 29.Warren DL, Hyde DM, Last JA. Synergistic interaction of ozone and respirable aerosols on rat lungs. IV. Protection by quenchers of reactive oxygen species. Toxicology. 1988;53:113–133. doi: 10.1016/0300-483x(88)90241-7. [DOI] [PubMed] [Google Scholar]
- 30.Last JA. Synergistic effects of air pollutants: Ozone plus a respirable aerosol. Research Report (Health effects Institute) 1991;38:1–32. [PubMed] [Google Scholar]
- 31.Kumarathasan P, Vincent R, Goegan P, Bjarnason S, Guenette J. Alteration in aromatic hydroxylation and lipid oxidation status in the lungs of rats exposed to ozone. Toxicol Mechan Methods. 2002;12:195–210. doi: 10.1080/15376520208951156. [DOI] [PubMed] [Google Scholar]
- 32.Fukunaga K, Nakazono N, Suzuki T, Takama K. Mechanism of oxidative damage to fish red blood cells by ozone. IUBMB life. 1999;48:631–634. doi: 10.1080/713803566. [DOI] [PubMed] [Google Scholar]
- 33.Mudd JB. Biochemical basis for the toxicity of ozone. In: Yunus M, Iqbal M, editors. Plant Response to Air Pollution. John Wiley and Sons Ltd; 1996. pp. 267–283. [Google Scholar]
- 34.Grimes HD, Perkins KK, Boss WF. Ozone degrades into hydroxyl radical under physiological conditions. A spin trapping study. Plant Physiol. 1983;72:1016–1020. doi: 10.1104/pp.72.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueno I, Hoshino M, Miura T, Shinriki N. Ozone exposure generates free radicals in the blood samples in vitro. Detection by the ESR spin-trapping technique. Free Radic Res. 1998;29:127–135. doi: 10.1080/10715769800300141. [DOI] [PubMed] [Google Scholar]
- 36.Ballinger CA, Cueto R, Squadrito G, Coffin JF, Velsor LW, Pryor WA, Postlethwait EM. Antioxidant-mediated augmentation of ozone-induced membrane oxidation. Free Radic Biol Medic. 2005;38:515–526. doi: 10.1016/j.freeradbiomed.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Kamofsky JR, Sima PD. Singlet oxygen generation from the reaction of ozone with plant leaves. J Biol Chem. 1995;270:7850–7852. doi: 10.1074/jbc.270.14.7850. [DOI] [PubMed] [Google Scholar]
- 38.Kamofsky JR, Sima PD. Singlet oxygen production from the reactions of ozone with biological molecules. J Biol Chem. 1991;266:9039–9042. [PubMed] [Google Scholar]
- 39.Kamofsky JR, Sima PD. Singlet-oxygen generation at gas-liquid interfaces: A significant artifact in the measurement of singlet-oxygen yields from ozone-biomolecule reactions. Photochem Photobiol. 1993;58:335–340. doi: 10.1111/j.1751-1097.1993.tb09570.x. [DOI] [PubMed] [Google Scholar]
- 40.Takeshita K, Olea-Azar CA, Mizuno M, Ozawa T. Singlet oxygen-dependent hydroxyl radical formation during uroporphyrin-mediated photosensitization in the presence of NADPH. Antioxidant Redox Signal. 2000;2:355–362. doi: 10.1089/ars.2000.2.2-355. [DOI] [PubMed] [Google Scholar]