Abstract
The Wnt signaling pathway is an ancient and evolutionarily conserved pathway that regulates crucial aspects of cell fate determination, cell migration, cell polarity, neural patterning and organogenesis during embryonic development. The Wnts are secreted glycoproteins and comprise a large family of nineteen proteins in humans hinting to a daunting complexity of signaling regulation, function and biological output. To date major signaling branches downstream of the Fz receptor have been identified including a canonical or Wnt/β-catenin dependent pathway and the non-canonical or β-catenin-independent pathway which can be further divided into the Planar Cell Polarity and the Wnt/Ca2+ pathways, and these branches are being actively dissected at the molecular and biochemical levels. In this review, we will summarize the most recent advances in our understanding of these Wnt signaling pathways and the role of these pathways in regulating key events during embryonic patterning and morphogenesis.
Key words: Wnt, frizzled, dishevelled, canonical, non-canonical, β-catenin, Planar Cell Polarity
Introduction
In the modern era of molecular medicine, much effort has been placed on dissecting the signaling pathways and molecular mechanisms that control the development of an organism. This effort is deeply engrained in the modern researcher and is singly directed towards the notion that understanding the mechanisms that control normal development can exponentially increase our hopes to prevent and treat the pleiotropic pathologies that arise when these mechanisms go awry. One key pathway that much effort has been placed in delineating is the Wnt signal transduction pathway.
The Wnt signaling pathway is a conserved pathway in metazoan animals. The name Wnt is resultant from a fusion of the name of the Drosophila segment polarity gene wingless and the name of the vertebrate homolog, integrated or int-1.1 The extra-cellular Wnt signal stimulates several intra-cellular signal transduction cascades, including the canonical or Wnt/β-catenin dependent pathway and the non-canonical or β-catenin-independent pathway which can be divided into the Planar Cell Polarity pathway and the Wnt/Ca2+ pathway.2 Wnt proteins regulate a dizzying array of cellular processes including cell fate determination, motility, polarity, primary axis formation and organogenesis and most recently, this pathway has been implicated in stem cell renewal. As the signaling pathways that play crucial role during embryogenesis are tightly regulated, the expression of the Wnt proteins and Wnt antagonists are exquisitely restricted both temporally and spatially during development.3 Deregulated Wnt signaling has catastrophic consequences for the developing embryo and it is now well appreciated that defective Wnt signaling is a causative factor for a number of pleiotropic human pathologies. Most notably, these pathologies include cancers of the breast, colon and skin, skeletal defects and human birth defect disorders including the most common human neural tube closure birth; spina bifida.4
Wnt proteins are secreted glycoproteins that bind to the N-terminal extra-cellular cysteine-rich domain of the Frizzled (Fz) receptor family of which there is ten Fz in humans.5 The Fz protein is a seven-transmembrane-span protein with topological homology to G-protein coupled receptors.6 In addition, to the interaction between Wnt and Fz, co-receptors are also required for mediating Wnt signaling. For example the low-density-lipoprotein-related protein5/6 (LRP5/6) is required to mediate the canonical Wnt signal.5 One key level of regulation of Wnt signaling occurs in the extra-cellular milieu with the presence of a diverse number of secreted Wnt antagonists.7 After binding of Wnt to the receptor complex, the signal is transduced to cytoplasmic phosphoprotein Dishevelled (Dsh/Dvl), and studies have uncovered that Dsh can directly interact with Fz.8 At the level of Dsh, the Wnt signal branches into at least three major cascades, canonical, Planar Cell Polarity and Wnt/Ca2+. Dsh is an important downstream component of this transduction pathway and is the first cytoplasmic protein that is pivotally involved in all three major branches of Wnt signaling. However, it still remains unclear how the Dsh protein regulates and channels signaling into each of these pathways. In this review we will focus on recent advances in our understanding of the molecular components of these major signaling branches, the various levels of regulation of signaling, and the distinct biological outcomes that are achieved. As a number of excellent reviews have been published, we will direct readers to these reviews at relevant points and encourage readers to visit the Wnt page which is an excellent resource for updated information at: www.stanford.edu/∼rnusse/wntwindow.html.
Wnt Secretion and Extra-Cellular Regulators
The Wnt ligands are secreted glycoproteins that are heavily modified prior to transport and release into the extra-cellular milieu. Studies have revealed that the Wnt proteins are glycosylated in the endoplasmic reticulum and also are palmitolated.9,10 The porcupine protein has been shown to play an important role in the palmitolation of the Wnt proteins, and their secretion is regulated by the wntless or evenness interrupted proteins and the retromer complex.11–14 In the extra-cellular matrix, the Wnt proteins may be bound to and stabilized by heparan sulfate proteoglycans including Dally and glypican 3 which further limits their diffusion and modulate their signaling abilities.15 In the extra-cellular matrix, a number of secreted proteins that bind to Wnts and prevent their interaction with either Fz or LRP5/6 to antagonize Wnt signaling have been identified. These include Dickkopf (Dkk) proteins,16 Wnt-inhibitor protein (WIF),17 soluble Frizzled-related proteins (SFRP),18 Cerebrus,19 Frzb20 and the context dependent Wnt inhibitor Wise.21 Each of these secreted inhibitors are tightly regulated during embryogenesis and serve to limit or likely create a gradient of Wnt signaling for pattern formation.22 An interesting recent finding is the identification of factors including Norrin23 and R-Spondin2,24 which can bind to the LRP5/6 receptor and may activate Wnt signaling independent of a Wnt ligand. The SOST protein can also bind to LRP5/6 where it can antagonize Wnt signaling.25
The Canonical Wnt Pathway
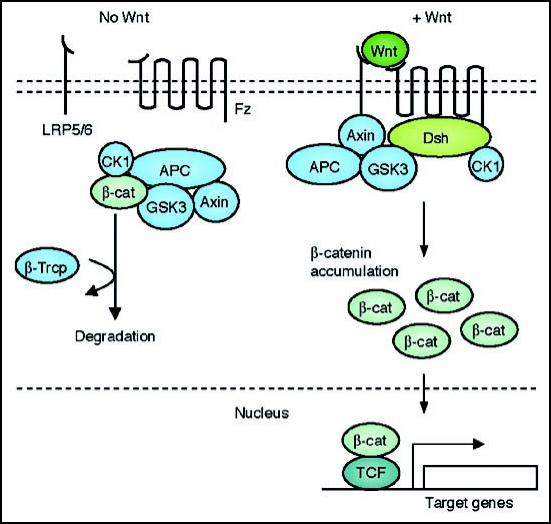
The canonical pathway was first identified and delineated from genetic screens in Drosophila and intensive studies in the fly, worm, frog, fish and mouse have led to the identification of a basic molecular signaling framework (Fig. 1). The hallmark of the canonical Wnt pathway is the accumulation and translocation of the adherens junction associated-protein β-catenin into the nucleus. Without Wnt signaling, cytoplasmic β-catenin is degraded by a β-catenin destruction complex, which includes Axin, adenomatosis polyposis coli (APC), protein phosphatase 2A (PP2A), glycogen synthase kinase 3 (GSK3) and casein kinase 1α (CK1α).5,26 Phosphorylation of β-catenin within this complex by Casein Kinase and GSK3 targets it for ubiquitination and subsequent proteolytic destruction by the proteosomal machinery.5 Binding of Wnt to its receptor complex composed of the Fz and the LRP5/6 triggers a series of events that disrupts the APC/Axin/GSK3 complex that is required for the targeted destruction of β-catenin.26 The binding of Wnt to the Fz/LRP5/6 complex induces the membrane translocation of a key negative regulator of signaling Axin, which binds to a conserved sequence in the cytoplasmic tail of LRP5/6.27,28 It is important to note that Wnt stimulation also regulates the stability of Axin for with Wnt stimulation a de-phosphorylation of Axin and a decrease in cytoplasmic levels of Axin has been documented.29–31 Recently it was reported that the microtubule actin cross-linking factor 1 (MACF1) might play an important role in this membrane translocation of Axin though the precise mechanism remains unclear.32,33 Upon membrane translocation of Axin, its binding to LRP5/6 is catalyzed by the phosphorylation of LRP5/6, mediated by either CK1γ or GSK3. An important point to realize is that CK1 and GSK3 appears to play distinct roles at two levels of canonical signaling; at the level of LRP5/6 their influence is positive, whereas at the level of β-catenin (see below), their roles are negative. The binding of Axin has been proposed to remove the negative activity of Axin on canonical Wnt signaling somehow leading to the activation of the phosphoprotein Dsh. Intriguingly, Dsh is also known to bind to Axin and Fz and it remains quite unresolved as to how Dsh becomes activated.34–36 Dsh is phosphorylated by a number of kinases including Casein Kinase 1,37 Casein Kinase 2,38 Metastasis Associated Kinase,39 Protein Kinase C40 and Par1.41 It is likely that this phosphorylation event regulates both the subcellular localization of Dsh and its ability to interact with effectors for the many branches of Wnt signaling.8 Dsh itself is a modular protein that contains three distinct domains, a DIX, a PDZ and a DEP domain and for canonical signaling, the DIX and PDZ domain appear to be central for mediating signaling.8 Once Dsh is activated, it inhibits the activity of the GSK3 enzyme, and activates a complex series of events that lead to the prevention of degradation of β-catenin and its consequent stabilization and accumulation in the cytoplasm.42 Stabilized β-catenin then translocates into the nucleus by a mechanism that remains poorly understood. β-catenin itself has no nuclear localization sequence (NLS), nor does its entry into the nucleus appear to require the function of the importin proteins or the Ran-mediated nuclear import.43 It has been proposed that β-catenin may likely “piggyback” with other factors to translocate into the nucleus and one of these may be Axin which appears to also undergo nuclear shuttling.44,45 For export of β-catenin, studies have identified two mechanisms for this process noting also that β-catenin does not possess a nuclear export sequence (NES), first an involvement of the Ran-binding protein3 (RanBP3) along with the APC protein which possesses an NES and another that is Ran-independent directly engaging proteins within the nuclear pore complex.46 In the nucleus, β-catenin exerts its effect on gene transcription by functioning as a transcriptional co-activator. A large number of binding partners for β-catenin in the nucleus has been uncovered and perhaps the best characterized are the members of the LEF/TCF DNA-binding transcription factors.47,48 This complex binds to the promoter of target genes. These target genes include those required for organizer formation during embryogenesis such as Siamois and Twin and genes involved in oncogenesis such as Myc and CyclinD1 during cancer formation.48,49 Other important binding partners of β-catenin also include Legless and Pygopus that influence the nuclear retention and transactivating ability of β-catenin for transcriptional regulation of its target genes.50–53
Figure 1.
A schematic representation of the canonical Wnt signal transduction cascade. Left, in the absence of Wnt ligand, a complex of Axin, APC, GSK3-β, CK1 and β-catenin is located in the cytosol. β-catenin is dually phosphorylated by CK1 and GSK3-β and targeted degraded by the proteosomal machinery mediated by β-TrCP. Right, with Wnt stimulation, signaling through the Fz receptor and LRP5/6 co-receptor complex induces the dual phosphorylation of LRP6 by CK1 and GSK3-β and this allows for the translocation of a protein complex containing Axin from the cytosol to the plasma membrane. Dsh is also recruited to the membrane and binds to Fz and Axin binds to phosphorylated LRP5/6. This complex formed at the membrane at Fz/LRP5/6 induces the stabilization of β-cat via either sequestration and/or degradation of Axin. β-catenin translocates into the nucleus where it complexes with Lef/Tcf family members to mediate transcriptional induction of target genes.
The canonical Wnt signaling plays a pivotal role in cell fate decisions during early embryogenesis, and perhaps the best model organism where this was elegantly worked out is the Xenopus system.49 After fertilization, the process of cortical rotation moves a dorsalizing factor to the future dorsal side of the embryo to establish the formation of the signaling center termed the Spemann-Mangold Organizer. Studies have uncovered that cortical rotation moves the Dsh protein and other components of the Wnt pathway leading to stabilization of β-catenin within the future dorsal side of the early Xenopus embryo, and is a critical event in the formation of the dorsal Spemann-Mangold Organizer. It was unclear whether a Wnt ligand was responsible for the establishment of the dorsal organizer or whether the Wnt signaling cascade was activated intra-cellularly without the need for a ligand.54 However, a recent study demonstrated that Wnt11 was indeed the Wnt ligand responsible for this action in early dorsal axis formation.55 In Xenopus embryos, the microinjection of RNA that activates canonical Wnt signaling can induce the formation of ectopic dorsal axial structures when injected ventrally. For example, overexpression of Xwnt8, Dsh, β-catenin or dominant-negative GSK3 can induce secondary axis formation.56 Furthermore, in early development of Xenopus, the β-catenin/TCF complex promotes transcription of Twin and Siamois, which encode homeodomain transcription factors. Twin and Siamois proteins are critical for the expression of organizer-specific genes.57,58 These data together cement the idea that the canonical pathway is required for dorsal axis formation during early development.
Following formation of the Spemann-Mangold Organizer, the canonical Wnt pathway also regulates anterior head formation and neural patterning. During gastrulation, a number of Wnt inhibitors including Cerberus, WIF, Dickkopf and Frzb are expressed in and secreted from the organizer to modulate formation of the anterior of the embryo and also promote anterior head formation.22 These inhibitory proteins can physically bind Wnt and block the formation of Wnt/Fz complex, resulting in low levels of nuclear β-catenin protein in the anterior region and a higher level within the posterior region of the gastrula embryo. The gradient of Wnt signal along anteroposterior axis is important for the formation of anterior head structure and neuroectodermal pattering.3 Canonical Wnt signaling also control subsequent posterior patterning and tail formation, as well as for formation of various organ systems including the heart, lungs, kidney, skin and bone.3,4,47,48,56 Wnt signaling also has recently been shown to play important roles in stem cell renewal.48 There seems to be no organ system that the canonical Wnt pathway does not directly or indirectly regulate formation of during embryo- genesis. This fact alone highlights the importance and crucial function of the canonical Wnt pathway.
The Non-Canonical Planar Cell Polarity Pathway
The non-canonical pathway is often referred to as the β-catenin-independent pathway and this pathway can be further divided into two distinct branches, the Planar Cell Polarity pathway or PCP pathway (Fig. 2) and the Wnt/Ca2+ pathway (Fig. 3). The PCP pathway emerged from genetic studies in Drosophila in which mutations in Wnt signaling components including Frizzled and Dishevelled were found to randomize the orientation of epithelial structures including cuticle hairs and sensory bristles.59 Cells in the epithelia are known to possess a defined apical-basolateral polarity but in addition they are also polarized along the plane of the epithelial layer. This rigid organization governs the orientation of structures including orientation of hair follicles, sensory bristles and hexagonal array of the ommatidia in the eye.60 In vertebrates, this organization has been shown to underlie the organization and orientation of stereo- cilia in the sensory epithelium of the inner ear, the organization of hair follicles, and the morphology and migratory behavior of dorsal mesodermal cells undergoing gastrulation.61 The defining feature of this pathway is its regulation of the actin cytoskeleton for such polarized organization of structures and directed migration. Moreover this pathway appears to function independently of transcription.
Figure 2.
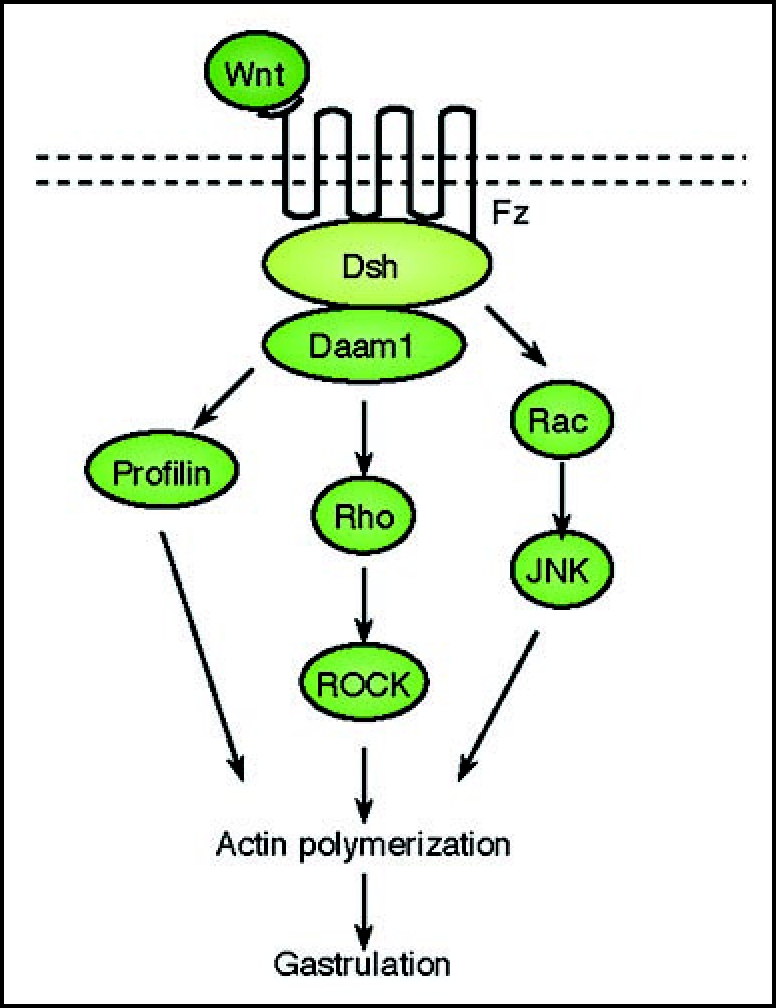
A schematic representation of the Planar Cell Polarity transduction cascade. Wnt signaling is transduced through Fz independent of LRP5/6 leading to the activation of Dsh. Dsh through Daam1 mediates activation of Rho which in turn activates Rho kinase (ROCK). Daam1 also mediates actin polymerization through the actin binding protein Profilin. Dsh also mediates activation of Rac, which in turn activates JNK. The signaling from Rock, JNK and Profilin are integrated for cytoskeletal changes for cell polarization and motility during gastrulation.
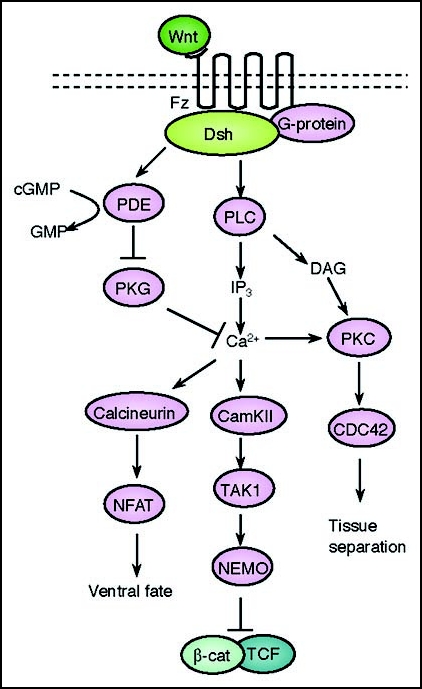
Figure 3.
A schematic representation of the Wnt/Ca2+ signal transduction cascade. Wnt signaling via Fz mediates activation of Dsh via activation of G-proteins. Dishevelled activates the phosphodiesterase PDE which inhibits PKG and in turn inhibits Ca2+ release. Dsh through PLC activates IP3, which leads to release of intracellular Ca2+, which in turn activates CamK11 and calcineurin. Calcineurin activate NF-AT to regulate ventral cell fates. CamK11 activates TAK and NLK, which inhibit β-catenin/TCF function to negatively regulate dorsal axis formation. DAG through PKC activates CDC42 to mediate tissue separation and cell movements during gastrulation.
Recent studies have shown that the non-canonical pathway is likely equivalent to the Planar Cell Polarity pathway in Drosophila because the core components of the pathways appear to be the same.60 The non-canonical pathway though a number of studies was found to regulate convergent extension movements during zebrafish and Xenopus gastrulation.62 During vertebrate gastrulation, mesodermal and ectodermal cells undergo convergent extension. In this processes, polarized cells intercalate along the mediolateral axis, resulting in mediolateral narrowing (convergent) and anteroposterior elongation (extension).63 The non-canonical Wnt pathway was shown to regulate both cell polarity and movements of dorsal mesodermal cells during convergent extension and later during neural tube closure.64 The Wnt4, Wnt5a and Wnt11 ligands have been established to signal via the non-canonical pathway, though recently Wnt11 has been shown to play a crucial role in early axis formation via the canonical pathway.55 Overexpression of these Wnts disrupted convergent extension in both Xenopus and zebrafish, without dramatically affecting cell fate determination regulated by the canonical pathway. Many other components of non-canonical pathway were also shown to disrupt convergent extension, including Dsh, Fz, Daam1, Rho, Rac, Prickle and Strabismus.65–74 Other factors such as PKA were shown to function as a negative regulator of non-canonical signaling. PKA can interact with RhoA and inhibit Rho activity, and also PKA does not affect β-catenin dependent gene transcription.75
In the non-canonical pathway, the Wnt signal is thought to be mediated through Fz independent from the LRP5/6 co-receptor.5 However, a recent study has shown that LRP6 can regulate convergent extension movements76 and more studies to address this question will be required. Importantly, thus far in Drosophila it is not clear whether a Wnt molecule regulates this pathway.59 The Fz co-receptors for the non-canonical pathway are not clearly defined even though there are putative candidates including NRH1,77 Ryk,78 PTK779 and ROR2.80 The signal is then transduced to Dsh, leading to its activation. The PDZ and DEP domains of Dsh are both utilized to activate two parallel pathways that activate the small GTPases Rho and Rac.8 For activation of the Rho branch of signaling, Wnt signaling induces a Dsh-Daam1 complex that leads to the activation of Daam1 and consequently activation of the Rho GTPase via at least one Rho guanine exchange factor identified thus far, WGEF.67,81 Daam1 (Dishevelled associated activator of morphogenesis 1) is a Formin-homology protein that can bind to Dsh and RhoA suggesting the possibility of a positive feedback loop for signaling.67 Activation of Rho GTPase leads to the activation of the Rho-associated kinase (ROCK)82 and myosin,83 which leads to modification of the actin cytoskeleton and cytoskeletal rearrangement. The second branch of signaling requires the DEP domain of Dsh and activates the Rac GTPase. This activation is independent of Daam1 and activated Rac in turn stimulates JNK activity.66,84 The factors downstream of JNK for non-canonical signaling remain poorly resolved. While both Rho and Rac have been implicated in transcriptional regulation, to date it is not clear whether any genes are transcribed downstream of these two GTPases for non-canonical signaling. The prevailing dogma remains that their primary roles are indeed solely for cytoskeletal modulation.63 It should be noted that how the activities of Rho and Rac, which are thought to have opposing functions, are coordinated for cell polarization and directional migration remains poorly defined.
While numerous experiments have confirmed that non-canonical signaling play an important role in convergent extension movement, how exactly this pathway regulates this process remains murky. In the PCP pathway in Drosophila, evidence has uncovered a key role in differential localization of key components including Fz, Flamingo (Fmi), Dsh, Strabismus and Prickle which mediates polarization.59 However, in vertebrates, differential localization has remained difficult to uncover due to a lack of quality reagents for examining this question. In dorsal migrating mesodermal cells, a differential localization of Dsh, Rac and PKCδ has been observed along the medial and lateral edges of the migratory cells in Xenopus but Prickle appears to be localized anteriorly (in zebrafish cells).62,85 The subcellular localization of Dsh remains one of the best analyzed. In cells undergoing convergent extension, Dsh is localized at the plasma membrane and accumulates in lamellipodia.86 In other cells, Dsh is located in the cytoplasm and possibly the nucleus where it may regulate canonical Wnt signaling.2,87 Overexpression or downregulation of Dsh was observed to disrupt membrane localization and mediolateral cell polarity. Thus, asymmetric and membrane accumulation of the Dsh protein and activation of PCP pathway seem to be required for convergent extension.88 It should also be noted that it remains unclear whether the non-canonical Wnt pathway plays an instructive or permissive role for gastrulation movements. The evidence in zebrafish demonstrated that injection of Wnt11 RNA into the two-cell stage embryo was sufficient to rescue the gastrulation defects observed in the silberblick mutant, indicating that localized expression of Wnt11 was not required and thus its role was permissive.89 Further studies will undoubtedly provide answers to this important question in the future.
The Non-canonical Wnt/Ca2+ Pathway
The second branch of the non-canonical Wnt signaling pathway is termed the Wnt/Ca2+ pathway and though this pathway shares a number of components of the Planar Cell Polarity pathway described previously, it has clear distinctions that allow it to be described as a separate branch (Fig. 3). This pathway further modulates canonical signaling for dorsal axis formation and Planar Cell Polarity signaling for gastrulation cell movements. The Wnt/Ca2+ pathway emerged with the finding that some Wnts and Fz receptors can stimulate intracellular Ca2+ release from ER and this pathway is dependent on G-proteins.90,91 Ca2+ waves in the embryo have further been demonstrated in regions of the zebrafish92 and Xenopus embryos93 undergoing gastrulation where it is thought to play crucial roles in early pattern formation. Wnt5a, Wnt11 and rat Fz2 (RFz-2) are capable of intracellular Ca2+ release, without affecting β-catenin stabilization.91 Calcium release by overexpression of Wnt5a or RFz-2 in zebrafish embryos is inhibited by pertusis toxin and the a subunit of transducin, which inhibit G protein signaling.94,95 These reports indicate that Wnt/Fz signaling leads to release of intracellular Ca2+ through trimeric G proteins. The calcium release and intracellular accumulation activates several Ca2+ sensitive proteins, including protein kinase C (PKC)96 and calcium/calmodulin-dependent kinase II (CamKII).97 CamK11 have been shown to activate the transcription factor NFAT to promote ventral cell fates in the Xenopus embryo.98 CamK11 has also been shown to activate TGFβ activated kinase (TAK1) and Nemo-like kinase (NLK) which can antagonize β-catenin/TCF signaling.99 Ca2+ can also activate PKC which regulates the process of tissue separation during gastrulation via activation of the small GTPase CDC42.100
The role of the Wnt/Ca2+ pathway during embryogenesis is diverse and includes the negative regulation of dorsal axis formation, promotion of ventral cell fate, regulation of tissue separation and convergent extension movements during gastrulation, and later in heart formation. In Xenopus embryos, calcium waves and release have been documented in cells undergoing during convergent extension and disruption of calcium release by pharmacological agents block convergent extension movements.90,91 The Wnt5a and Wnt11 ligands can induce Ca2+ release and activate PKC and CamKII. The inhibition of convergent extension movements induced by misexpression of Wnt5a can be partially rescued by expression of CamKII in zebrafish embryos.101 In addition, overexpression of CamKII itself can inhibit gastrulation movements in Xenopus embryos.97 The Dsh protein via the PDZ and DEP domains can also induce Ca2+ release and activation of PKC and CamK11 in order to regulate heart formation, suggesting that it is a crucial component of the Wnt/Ca2+ pathway.96 Together, these studies show that the Wnt/Ca2+ pathway functions as a critical modulator of both the canonical and Planar Cell Polarity pathways. In the future, it will be important to understand how this pathway functions at the molecular level to regulate the diverse biological outcomes of these two pathways.
Other Wnt Signaling Pathways
While the canonical, Planar Cell Polarity and Wnt/Ca2+ pathways remain the most studied branches to date, a number of other pathways have been emerging. These pathways may share overlapping components of the Planar Cell Polarity and Wnt/Ca2+ branches but appear to have distinct outcomes. As such their relationship to these branches remains currently obscure but we will briefly describe what is known about these pathways. During neuronal migration and synaptogenesis, Wnt signaling has been shown to regulate the microtubule cytoskeleton and this pathway relies on the activity of GSK3 and Dsh.102,103 During axonal guidance, another Wnt pathway via the Ryk receptor and proto-oncoprotein Src regulates repulsion of neurons.104,105 During gastrulation, Wnt signaling via casein kinase inhibits the Rap1-GTPase leading to cytoskeletal modulation.106,107 For cell growth, Wnt signaling via Dsh and GSK3 regulates the TSC2 tumor suppressor to negatively regulate mTor.108,109 For epithelial polarity and cell migration, Wnt signaling via Dsh, aPKC, Par3, Par6 and LGl modulates cell polarity and the microtubule cytoskeleton.110 During myogenesis, Wnt signaling via PKA and the transcription factor CREB modulates gene transcription of muscle specific genes including MyoD and Myf5.111 During gastrulation, Wnt signaling via the ROR2 receptor modulates expression of the proto-cadherin PAPC via a CDC42 and JNK pathway.112 Together these emerging pathways hint to the complexity by which Wnt signaling may exert effects on a number of developing cellular systems and studies to understand how these pathways are integrated remain crucial.
Signal Specificity
The large number of Wnt ligands in vertebrates along with the many branches of signaling that are triggered by Wnt ligands have led to the question of whether a specific Wnt ligand may selectively activate one particular branch of signaling. However, the answer to this question remains unresolved since some Wnt ligands can activate both canonical and non-canonical pathways.7,64,113 For example, Wnt8 can lead to the accumulation of nuclear β-catenin but it does not activate Wnt/Ca2+ signaling and Wnt11 thus far can activate both canonical and non-canonical signaling.55 Likewise, Wnt5a can activate the non-canonical pathway and induce calcium flux but not activate β-catenin stabilization.91,114 Similarly, the Frizzled receptors appear to activate both canonical and non-canonical pathways precluding a direct classification of these proteins into either specific activators or canonical or non-canonical signaling.4,6,7 However, it must be noted that some studies have reported that some Fz receptors may have preference for the Wnt/Ca2+ branch of signaling. The misexpression of rat frizzled-1 (RFz-1) in zebrafish embryos did not increase Ca2+ release and activation of PKC and CamKII. On the other hand, RFz-2 stimulates CamKII activity and induces PKC translocation.6,115 Thus, it is still unclear how different Wnt ligands or Frizzled receptors are able to deploy specific pathway activation. The current dogma appears to be that the specificity of a particular pathway may not be dictated by a specific ligand/receptor combination per se. It is reasonable to propose and several studies have shown that co-receptors, binding partners of Dsh and/or subcellular localization of Dsh are likely the important factors that may dictate signal specificity.5,8 For example, LRP-5 and LRP-6 are implicated in only the canonical pathway,5,8 while NRH1, PTK7 and ROR2 selectively transduce the non-canonical pathway.8,113
The Dsh protein is the first intracellular component that binds to Fz and functions in all branches of signaling. The Dsh protein, as previously mentioned has three conserved domains: DIX, PDZ and DEP domains116 and it appears that these specific domains are utilized for pathway specific signaling.8 The DIX domain of Dsh binds to Axin, which is a component of β-catenin destruction complex, and inhibits complex formation and the DIX domain is essential for canonical pathway but not required for non-canonical pathway. The PDZ domain of Dsh function in the canonical, non-canonical and Wnt/ Ca2+ pathway while the DEP domain functions in non-canonical signaling.8,35 The DEP domain is also required for membrane translocation of Dsh.117 In addition to the use of distinct domains, the specific pathway that Dsh activates depends on its binding partners. Complexity of this signaling can be seen in the thirty or so binding partners identified to date and we can expect more continue to be added in the future.8 The components of non-canonical signaling, such as Daam1, Strabismus and Prickle can bind to PDZ domain of Dsh and function in PCP pathway.8 Conversely, the Dsh that binds to CK1, GSK3 and GBP functions in canonical signaling.5,7
The subcellular localization of Dsh is also important for specificity of signaling. As mentioned above, asymmetric membrane accumulation of Dsh is important for the non-canonical pathway and convergent extension movements.118–120 Removal of Dsh from the membrane inhibits Rho activation, resulting in disruption of convergent extension but does not impair canonical signaling.88 Although recent studies have showed that Dsh shuttles between the cytoplasm and nucleus, the importance of Dsh function in the nucleus remains unclear.2,87
Conclusion
The recent evidence to date has strongly cemented the fact that Wnt signaling plays a critical role in pattern formation during embryogenesis. Many studies over the last two decades have identified numerous signaling components that have helped to build a molecular framework for the many branches of the Wnt signal transduction pathway. However, the diverse function, integration and specificity of the Wnt signaling are still unclear. Furthermore, we lack a clear understanding of many of the biochemical aspects within this signaling framework. Recent studies have demonstrated a strong correlation and at times causative relationships between deregulated Wnt signaling and human diseases. Thus the investigation of Wnt signaling remains an important goal for dually understanding both the basic mechanism of embryonic development and human diseases. Undoubtedly, the future holds many important breakthrough studies in Wnt signaling that will further our understanding of this important pathway and we all await these discoveries with eager enthusiasm.
Acknowledgements
We are grateful to members of the Habas laboratory for discussion and critical comments and R.H. is supported by grants from the American Heart Association, the March of Dimes, the National Science Foundation and the National Institutes of Health.
Footnotes
Previously published online as an Organogenesis E-publication: http://www.landesbioscience.com/journals/organogenesis/article/5851
References
- 1.Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- 2.Habas R, Dawid IB. Dishevelled and Wnt signaling: is the nucleus the final frontier? J Biol. 2005;4:2. doi: 10.1186/jbiol22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamaguchi TP. Heads or tails: Wnts and anterior-posterior patterning. Curr Biol. 2001;11:713–724. doi: 10.1016/s0960-9822(01)00417-1. [DOI] [PubMed] [Google Scholar]
- 4.Logan CY, Nusse R. The Wnt Signaling Pathway in Development and Disease. Annu Rev Cell Dev Biol. 2004 doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 5.He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 6.Schulte G, Bryja V. The Frizzled family of unconventional G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:518–525. doi: 10.1016/j.tips.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. J Cell Sci. 2006;119:395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- 8.Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–4436. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- 9.Smolich BD, McMahon JA, McMahon AP, Papkoff J. Wnt family proteins are secreted and associated with the cell surface. Mol Biol Cell. 1993;4:1267–1275. doi: 10.1091/mbc.4.12.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 11.Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 12.Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Hausmann G, Banziger C, Basler K. Helping Wingless take flight: how WNT proteins are secreted. Nat Rev Mol Cell Biol. 2007;8:331–336. doi: 10.1038/nrm2141. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann K. A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling. Trends Biochem Sci. 2000;25:111–112. doi: 10.1016/s0968-0004(99)01539-x. [DOI] [PubMed] [Google Scholar]
- 15.Lin X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development. 2004;131:6009–6021. doi: 10.1242/dev.01522. [DOI] [PubMed] [Google Scholar]
- 16.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 17.Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB, Nathans J. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999;398:431–436. doi: 10.1038/18899. [DOI] [PubMed] [Google Scholar]
- 18.Hoang BH, Thomas JT, Abdul-Karim FW, Correia KM, Conlon RA, Luyten FP, Ballock RT. Expression pattern of two Frizzled-related genes, Frzb-1 and Sfrp-1, during mouse embryogenesis suggests a role for modulating action of Wnt family members. Dev Dyn. 1998;212:364–372. doi: 10.1002/(SICI)1097-0177(199807)212:3<364::AID-AJA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 19.Bouwmeester T, Kim S, Sasai Y, Lu B, De Robertis EM. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann's organizer. Nature. 1996;382:595–601. doi: 10.1038/382595a0. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Krinks M, Lin K, Luyten FP, Moos M., Jr Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell. 1997;88:757–766. doi: 10.1016/s0092-8674(00)81922-4. [DOI] [PubMed] [Google Scholar]
- 21.Itasaki N, Jones CM, Mercurio S, Rowe A, Domingos PM, Smith JC, Krumlauf R. Wise, a context-dependent activator and inhibitor of Wnt signalling. Development. 2003;130:4295–4305. doi: 10.1242/dev.00674. [DOI] [PubMed] [Google Scholar]
- 22.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 23.Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, Nathans J. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- 24.Kazanskaya O, Glinka A, del Barco Barrantes I, Stannek P, Niehrs C, Wu W. R-Spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for Xenopus myogenesis. Dev Cell. 2004;7:525–534. doi: 10.1016/j.devcel.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 25.Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280:26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 26.Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- 27.Mao J, Wang J, Liu B, Pan W, Farr GH, 3rd, Flynn C, Yuan H, Takada S, Kimelman D, Li L, Wu D. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7:801–809. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 28.Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, Almeida K, Wang J, Doble B, Woodgett J, Wynshaw-Boris A, Hsieh JC, He X. Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development. 2008;135:367–375. doi: 10.1242/dev.013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tolwinski NS, Wehrli M, Rives A, Erdeniz N, DiNardo S, Wieschaus E. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev Cell. 2003;4:407–418. doi: 10.1016/s1534-5807(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 30.Willert K, Logan CY, Arora A, Fish M, Nusse R. A Drosophila Axin homolog, Daxin, inhibits Wnt signaling. Development. 1999;126:4165–4173. doi: 10.1242/dev.126.18.4165. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto H, Kishida S, Kishida M, Ikeda S, Takada S, Kikuchi A. Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. J Biol Chem. 1999;274:10681–10684. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- 32.Chen HJ, Lin CM, Lin CS, Perez-Olle R, Leung CL, Liem RK. The role of microtubule actin cross-linking factor 1 (MACF1) in the Wnt signaling pathway. Genes Dev. 2006;20:1933–1945. doi: 10.1101/gad.1411206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Habas R. Canonical Wnt signaling: an unexpected new player. Dev Cell. 2006;11:138–139. doi: 10.1016/j.devcel.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Kishida S, Yamamoto H, Hino S, Ikeda S, Kishida M, Kikuchi A. DIX domains of Dvl and axin are necessary for protein interactions and their ability to regulate beta-catenin stability. Mol Cell Biol. 1999;19:4414–4422. doi: 10.1128/mcb.19.6.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wharton KA., Jr Runnin' with the Dvl: proteins that associate with Dsh/Dvl and their significance to Wnt signal transduction. Dev Biol. 2003;253:1–17. doi: 10.1006/dbio.2002.0869. [DOI] [PubMed] [Google Scholar]
- 36.Wong HC, Bourdelas A, Krauss A, Lee HJ, Shao Y, Wu D, Mlodzik M, Shi DL, Zheng J. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol Cell. 2003;12:1251–1260. doi: 10.1016/s1097-2765(03)00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peters JM, McKay RM, McKay JP, Graff JM. Casein kinase I transduces Wnt signals. Nature. 1999;401:345–350. doi: 10.1038/43830. [DOI] [PubMed] [Google Scholar]
- 38.Willert K, Brink M, Wodarz A, Varmus H, Nusse R. Casein kinase 2 associates with and phosphorylates dishevelled. EMBO J. 1997;16:3089–3096. doi: 10.1093/emboj/16.11.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kibardin A, Ossipova O, Sokol SY. Metastasis-associated kinase modulates Wnt signaling to regulate brain patterning and morphogenesis. Development. 2006;133:2845–2854. doi: 10.1242/dev.02445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen W, ten Berge D, Brown J, Ahn S, Hu LA, Miller WE, Caron MG, Barak LS, Nusse R, Lefkowitz RJ. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 2003;301:1391–1394. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- 41.Ossipova O, Dhawan S, Sokol S, Green JB. Distinct PAR-1 proteins function in different branches of Wnt signaling during vertebrate development. Dev Cell. 2005;8:829–841. doi: 10.1016/j.devcel.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Hatsell S, Rowlands T, Hiremath M, Cowin P. Beta-catenin and Tcfs in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2003;8:145–158. doi: 10.1023/a:1025944723047. [DOI] [PubMed] [Google Scholar]
- 43.Fagotto F, Gluck U, Gumbiner BM. Nuclear localization signal-independent and importin/karyopherin-independent nuclear import of beta-catenin. Curr Biol. 1998;8:181–190. doi: 10.1016/s0960-9822(98)70082-x. [DOI] [PubMed] [Google Scholar]
- 44.Cong F, Varmus H. Nuclear-cytoplasmic shuttling of Axin regulates subcellular localization of beta-catenin. Proc Natl Acad Sci USA. 2004;101:2882–2887. doi: 10.1073/pnas.0307344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwarz-Romond T, Metcalfe C, Bienz M. Dynamic recruitment of axin by Dishevelled protein assemblies. J Cell Sci. 2007;120:2402–2412. doi: 10.1242/jcs.002956. [DOI] [PubMed] [Google Scholar]
- 46.Hendriksen J, Fagotto F, van der Velde H, van Schie M, Noordermeer J, Fornerod M. RanBP3 enhances nuclear export of active (beta)-catenin independently of CRM1. J Cell Biol. 2005;171:785–797. doi: 10.1083/jcb.200502141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 48.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 49.Harland R, Gerhart J. Formation and function of Spemann's organizer. Annu Rev Cell Dev Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- 50.Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, Murone M, Zullig S, Basler K. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell. 2002;109:47–60. doi: 10.1016/s0092-8674(02)00679-7. [DOI] [PubMed] [Google Scholar]
- 51.Parker DS, Jemison J, Cadigan KM. Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila. Development. 2002;129:2565–2576. doi: 10.1242/dev.129.11.2565. [DOI] [PubMed] [Google Scholar]
- 52.Thompson B, Townsley F, Rosin-Arbesfeld R, Musisi H, Bienz M. A new nuclear component of the Wnt signalling pathway. Nat Cell Biol. 2002;4:367–373. doi: 10.1038/ncb786. [DOI] [PubMed] [Google Scholar]
- 53.Townsley FM, Cliffe A, Bienz M. Pygopus and Legless target Armadillo/beta-catenin to the nucleus to enable its transcriptional co-activator function. Nat Cell Biol. 2004;6:626–633. doi: 10.1038/ncb1141. [DOI] [PubMed] [Google Scholar]
- 54.Jessen JR, Solnica-Krezel L. Axis formation—beta-catenin catches a Wnt. Cell. 2005;120:736–737. doi: 10.1016/j.cell.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 55.Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X, Heasman J. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120:857–871. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 56.De Robertis EM, Larrain J, Oelgeschlager M, Wessely O. The establishment of Spemann's organizer and patterning of the vertebrate embryo. Nat Rev Genet. 2000;1:171–181. doi: 10.1038/35042039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Robertis EM. Spemann's organizer and self-regulation in amphibian embryos. Nat Rev Mol Cell Biol. 2006;7:296–302. doi: 10.1038/nrm1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8:126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- 60.Mlodzik M. Planar cell polarization: do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet. 2002;18:564–571. doi: 10.1016/s0168-9525(02)02770-1. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- 62.Wallingford JB, Fraser SE, Harland RM. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- 63.Keller R, Davidson LA, Shook DR. How we are shaped: the biomechanics of gastrulation. Differentiation. 2003;71:171–205. doi: 10.1046/j.1432-0436.2003.710301.x. [DOI] [PubMed] [Google Scholar]
- 64.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 65.Djiane A, Riou J, Umbhauer M, Boucaut J, Shi D. Role of frizzled 7 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2000;127:3091–3100. doi: 10.1242/dev.127.14.3091. [DOI] [PubMed] [Google Scholar]
- 66.Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 68.Park M, Moon RT. The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nat Cell Biol. 2002;4:20–25. doi: 10.1038/ncb716. [DOI] [PubMed] [Google Scholar]
- 69.Sokol SY. Analysis of Dishevelled signalling pathways during Xenopus development. Curr Biol. 1996;6:1456–1467. doi: 10.1016/s0960-9822(96)00750-6. [DOI] [PubMed] [Google Scholar]
- 70.Tahinci E, Symes K. Distinct functions of Rho and Rac are required for convergent extension during Xenopus gastrulation. Dev Biol. 2003;259:318–335. doi: 10.1016/s0012-1606(03)00206-9. [DOI] [PubMed] [Google Scholar]
- 71.Takeuchi M, Nakabayashi J, Sakaguchi T, Yamamoto TS, Takahashi H, Takeda H, Ueno N. The prickle-related gene in vertebrates is essential for gastrulation cell movements. Curr Biol. 2003;13:674–679. doi: 10.1016/s0960-9822(03)00245-8. [DOI] [PubMed] [Google Scholar]
- 72.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 73.Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, Solnica-Krezel L. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol. 2002;4:610–615. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carreira-Barbosa F, Concha ML, Takeuchi M, Ueno N, Wilson SW, Tada M. Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development. 2003;130:4037–4046. doi: 10.1242/dev.00567. [DOI] [PubMed] [Google Scholar]
- 75.Park E, Kim GH, Choi SC, Han JK. Role of PKA as a negative regulator of PCP signaling pathway during Xenopus gastrulation movements. Dev Biol. 2006;292:344–457. doi: 10.1016/j.ydbio.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 76.Tahinci E, Thorne CA, Franklin JL, Salic A, Christian KM, Lee LA, Coffey RJ, Lee E. Lrp6 is required for convergent extension during Xenopus gastrulation. Development. 2007;134:4095–4106. doi: 10.1242/dev.010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sasai N, Nakazawa Y, Haraguchi T, Sasai Y. The neurotrophin-receptor-related protein NRH1 is essential for convergent extension movements. Nat Cell Biol. 2004;6:741–748. doi: 10.1038/ncb1158. [DOI] [PubMed] [Google Scholar]
- 78.Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 79.Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- 80.Nishita M, Yoo SK, Nomachi A, Kani S, Sougawa N, Ohta Y, Takada S, Kikuchi A, Minami Y. Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J Cell Biol. 2006;175:555–562. doi: 10.1083/jcb.200607127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tanegashima K, Zhao H, Dawid IB. WGEF activates Rho in the Wnt-PCP pathway and controls convergent extension in Xenopus gastrulation. EMBO J. 2008;27:606–617. doi: 10.1038/emboj.2008.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marlow F, Topczewski J, Sepich D, Solnica-Krezel L. Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr Biol. 2002;12:876–884. doi: 10.1016/s0960-9822(02)00864-3. [DOI] [PubMed] [Google Scholar]
- 83.Weiser DC, Pyati UJ, Kimelman D. Gravin regulates mesodermal cell behavior changes required for axis elongation during zebrafish gastrulation. Genes Dev. 2007;21:1559–1571. doi: 10.1101/gad.1535007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li L, Yuan H, Xie W, Mao J, Caruso AM, McMahon A, Sussman DJ, Wu D. Dishevelled proteins lead to two signaling pathways. Regulation of LEF-1 and c-Jun N-terminal kinase in mammalian cells. J Biol Chem. 1999;274:129–134. doi: 10.1074/jbc.274.1.129. [DOI] [PubMed] [Google Scholar]
- 85.Solnica-Krezel L. Conserved patterns of cell movements during vertebrate gastrulation. Curr Biol. 2005;15:213–228. doi: 10.1016/j.cub.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 86.Wallingford JB, Harland RM. Xenopus Dishevelled signaling regulates both neural and mesodermal convergent extension: parallel forces elongating the body axis. Development. 2001;128:2581–2592. doi: 10.1242/dev.128.13.2581. [DOI] [PubMed] [Google Scholar]
- 87.Itoh K, Brott BK, Bae GU, Ratcliffe MJ, Sokol SY. Nuclear localization is required for Dishevelled function in Wnt/β-catenin signalling. J Biol. 2005;4:3. doi: 10.1186/jbiol20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park TJ, Gray RS, Sato A, Habas R, Wallingford JB. Subcellular localization and signaling properties of dishevelled in developing vertebrate embryos. Curr Biol. 2005;15:1039–1044. doi: 10.1016/j.cub.2005.04.062. [DOI] [PubMed] [Google Scholar]
- 89.Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- 90.Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 91.Slusarski DC, Pelegri F. Calcium signaling in vertebrate embryonic patterning and morphogenesis. Dev Biol. 2007;307:1–13. doi: 10.1016/j.ydbio.2007.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gilland E, Miller AL, Karplus E, Baker R, Webb SE. Imaging of multicellular large-scale rhythmic calcium waves during zebrafish gastrulation. Proc Natl Acad Sci USA. 1999;96:157–161. doi: 10.1073/pnas.96.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wallingford JB, Ewald AJ, Harland RM, Fraser SE. Calcium signaling during convergent extension in Xenopus. Curr Biol. 2001;11:652–661. doi: 10.1016/s0960-9822(01)00201-9. [DOI] [PubMed] [Google Scholar]
- 94.Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997;390:410–413. doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- 95.Slusarski DC, Yang-Snyder J, Busa WB, Moon RT. Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev Biol. 1997;182:114–120. doi: 10.1006/dbio.1996.8463. [DOI] [PubMed] [Google Scholar]
- 96.Sheldahl LC, Slusarski DC, Pandur P, Miller JR, Kuhl M, Moon RT. Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J Cell Biol. 2003;161:769–777. doi: 10.1083/jcb.200211094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kuhl M, Sheldahl LC, Malbon CC, Moon RT. Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J Biol Chem. 2000;275:12701–12711. doi: 10.1074/jbc.275.17.12701. [DOI] [PubMed] [Google Scholar]
- 98.Saneyoshi T, Kume S, Amasaki Y, Mikoshiba K. The Wnt/calcium pathway activates NF-AT and promotes ventral cell fate in Xenopus embryos. Nature. 2002;417:295–299. doi: 10.1038/417295a. [DOI] [PubMed] [Google Scholar]
- 99.Ishitani T, Ninomiya-Tsuji J, Nagai S, Nishita M, Meneghini M, Barker N, Waterman M, Bowerman B, Clevers H, Shibuya H, Matsumoto K. The TAK1-NLK-MAPK-related pathway antagonizes signalling between beta-catenin and transcription factor TCF. Nature. 1999;399:798–802. doi: 10.1038/21674. [DOI] [PubMed] [Google Scholar]
- 100.Winklbauer R, Medina A, Swain RK, Steinbeisser H. Frizzled-7 signalling controls tissue separation during Xenopus gastrulation. Nature. 2001;413:856–860. doi: 10.1038/35101621. [DOI] [PubMed] [Google Scholar]
- 101.Westfall TA, Brimeyer R, Twedt J, Gladon J, Olberding A, Furutani-Seiki M, Slusarski DC. Wnt-5/pipetail functions in vertebrate axis formation as a negative regulator of Wnt/beta-catenin activity. J Cell Biol. 2003;162:889–898. doi: 10.1083/jcb.200303107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Salinas PC. Wnt factors in axonal remodelling and synaptogenesis. Biochem Soc Symp. 1999;65:101–109. [PubMed] [Google Scholar]
- 103.Salinas PC. Modulation of the microtubule cytoskeleton: a role for a divergent canonical Wnt pathway. Trends Cell Biol. 2007;17:333–342. doi: 10.1016/j.tcb.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 104.Schmitt AM, Shi J, Wolf AM, Lu CC, King LA, Zou Y. Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature. 2006;439:31–37. doi: 10.1038/nature04334. [DOI] [PubMed] [Google Scholar]
- 105.Zou Y. Wnt signaling in axon guidance. Trends Neurosci. 2004;27:528–532. doi: 10.1016/j.tins.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 106.Habas R, He X. Cell signaling: moving to a Wnt-Rap. Curr Biol. 2007;17:474–477. doi: 10.1016/j.cub.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 107.Tsai IC, Amack JD, Gao ZH, Band V, Yost HJ, Virshup DM. A Wnt-CKIvarepsilon-Rap1 Pathway Regulates Gastrulation by Modulating SIPA1L1, a Rap GTPase Activating Protein. Dev Cell. 2007;12:335–347. doi: 10.1016/j.devcel.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Choo AY, Roux PP, Blenis J. Mind the GAP: Wnt steps onto the mTORC1 train. Cell. 2006;126:834–836. doi: 10.1016/j.cell.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 109.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 110.Dollar GL, Weber U, Mlodzik M, Sokol SY. Regulation of Lethal giant larvae by Dishevelled. Nature. 2005;437:1376–1380. doi: 10.1038/nature04116. [DOI] [PubMed] [Google Scholar]
- 111.Chen AE, Ginty DD, Fan CM. Protein kinase A signalling via CREB controls myogenesis induced by Wnt proteins. Nature. 2005;433:317–322. doi: 10.1038/nature03126. [DOI] [PubMed] [Google Scholar]
- 112.Schambony A, Wedlich D. Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev Cell. 2007;12:779–792. doi: 10.1016/j.devcel.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 113.Endo Y, Rubin JS. Wnt signaling and neurite outgrowth: insights and questions. Cancer Sci. 2007;98:1311–1317. doi: 10.1111/j.1349-7006.2007.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pandur P, Maurus D, Kuhl M. Increasingly complex: new players enter the Wnt signaling network. Bioessays. 2002;24:881–884. doi: 10.1002/bies.10164. [DOI] [PubMed] [Google Scholar]
- 115.Huang HC, Klein PS. The Frizzled family: receptors for multiple signal transduction pathways. Genome Biol. 2004;5:234. doi: 10.1186/gb-2004-5-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boutros M, Mlodzik M. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech Dev. 1999;83:27–37. doi: 10.1016/s0925-4773(99)00046-5. [DOI] [PubMed] [Google Scholar]
- 117.Rothbacher U, Laurent MN, Deardorff MA, Klein PS, Cho KW, Fraser SE. Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO J. 2000;19:1010–1022. doi: 10.1093/emboj/19.5.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- 119.Rosin-Arbesfeld R, Townsley F, Bienz M. The APC tumour suppressor has a nuclear export function. Nature. 2000;406:1009–1012. doi: 10.1038/35023016. [DOI] [PubMed] [Google Scholar]
- 120.Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]