Abstract
Wnt/β-catenin signaling has come to the forefront of liver biology in recent years. This pathway regulates key pathophysiological events inherent to the liver including development, regeneration and cancer, by dictating several biological processes such as proliferation, apoptosis, differentiation, adhesion, zonation and metabolism in various cells of the liver. This review will examine the studies that have uncovered the relevant roles of Wnt/β-catenin signaling during the process of liver development. We will discuss the potential roles of Wnt/β-catenin signaling during the phases of development, including competence, hepatic induction, expansion and morphogenesis. In addition, we will discuss the role of negative and positive regulation of this pathway and how the temporal expression of Wnt/β-catenin can direct key processes during hepatic development. We will also identify some of the major deficits in the current understanding of the role of Wnt/β-catenin signaling in liver development in order to provide a perspective for future studies. Thus, this review will provide a contextual overview of the role of Wnt/β-catenin signaling during hepatic organogenesis.
Key words: liver development, liver cancer, liver regeneration, Wnt signaling, proliferation, differentiation
The Wnt/β-catenin pathway is an evolutionarily well-conserved pathway that has proven to be essential to normal cellular processes such as development, growth, survival, regeneration and self-renewal.1–5 Its diverse functions also include the initiation and progression of cancer.6 In fact, one area in which this pathway has been extensively studied is in liver cancer.
Mutations of Wnt/β-catenin pathway members in hepatocarcinogenesis are common. For example, 90–100% of hepatoblastomas contain mutations in adenomatous polyposis coli (APC), CTNNB1 and/or Axin1/2, which causes cytoplasmic and nuclear localization of β-catenin.7–9 Axin1 and β-catenin mutations have also been identified in approximately 25% of hepatocellular carcinomas,10–12 while overexpression of the frizzled-7 receptor13 and glycogen synthase kinase-3 (GSK-3) inactivation14 can also lead to aberrant β-catenin pathway activation. The dysregulation of this pathway in hepatic cancers makes it an attractive target for potential therapies, and experimental treatment in vivo has shown promising results. For example, inhibiting β-catenin expression by siRNA or R-Etodolac decreases proliferation and survival of human hepatoma cell lines.15,16 Since cancer recapitulates development, determining the timing of β-catenin activation during hepatogenesis will help us to better understand the inappropriate activation of this pathway in hepatocarcinogenesis.
Recent work has elucidated the role of β-catenin signaling in the liver, and has highlighted its essential role in liver health and disease.17 In addition, emerging evidence suggests that this pathway plays a key role in liver organogenesis.
The Wnt/β-Catenin Pathway
The Wnt/β-catenin pathway is inactive in normal unstimulated cells. In this steady-state condition, β-catenin, the central player in this signaling cascade, is bound in a complex with Axin, APC and GSK3. In the absence of Wnt, β-catenin is phosphorylated by casein kinase 1 (CK1) and GSK3α/β at serine/threonine residues located at the N-terminal region of the protein.18 This phosphorylation targets β-catenin for ubiquitination and ultimate degradation by the proteasome. When Wnt proteins bind to the Frizzled receptor on the surface of cells, it activates the canonical Wnt pathway. The Wnt/Frizzled interaction induces association with the low-density lipoprotein receptor related protein (LRP) 5/6, and this complex then recruits Dishevelled, which is thought to inactivate GSKβ.19 Inactivation of GSKβ leads to the absence of β-catenin phosphorylation, which subsequently releases it from the Axin/APC/GSK3 complex. β-catenin then translocates to the nucleus, where it binds to lymphoid enhancer-binding factor 1/T cell-specific transcription factor (LEF/TCF), displaces the transcriptional inhibitor Groucho, and in complex with TCF activates target genes important in proliferation and differentiation.3
In addition to the above-mentioned participants involved in the canonical Wnt signaling pathway, several other proteins are known to interact with β-catenin. E-cadherin, along with α-catenin, forms a complex with β-catenin at the surface of hepatocytes.20 α-catenin binds to actin, anchoring the complex to the cytoskeleton. The β-catenin/E-cadherin interaction also mediates cell-cell adhesion and is regulated by the phosphorylation of β-catenin at a specific tyrosine residue (Y654).21 Specifically in liver, this causes dissociation of the complex and subsequent degradation of E-cadherin, resulting in a loss of adherens junctions and impaired apical trafficking in hepatocytes.22 Loss of adhesion may also contribute to motility, which is an important component of the cellular response in processes such as development, regeneration and cancer growth.
TGFβ, a known regulator of self-renewal which has also been implicated in the modulation of hepatocellular carcinoma,23 plays an important role in E-cadherin/β-catenin interactions as well. TGFβ-mediated loss of E-cadherin results in the release of β-catenin from cell-cell contacts and its subsequent translocation to the cytoplasm. Active β-catenin thus leads to the increased cell motility and invasive phenotype seen in gastrointestinal and liver cancers.24,25
Another important interaction involving β-catenin in hepatocytes is the β-catenin/Met complex. Met is the receptor for hepatocyte growth factor (HGF), a known mitogen, motogen and morphogen for the liver.26 We have previously shown that Met and β-catenin associate at the surface of hepatocytes; binding of HGF to its receptor induces phosphorylated Met to phosphorylate β-catenin, which results in its translocation to the nucleus.27 Another study by our lab found that injecting the human HGF gene into mice leads to hepatomegaly via dissociation of the Met/β-catenin complex and induction of the β-catenin pathway.28 β-catenin also regulates HGF-induced cell morphogenesis,29 and interactions between Met and a mutated active form of β-catenin have been found to facilitate hepatocellular carcinoma.30 The Met/β-catenin pathways thus cooperate to induce hepatocyte proliferation both in vitro and in vivo.
This review will discuss these pathways and interactions—Wnt/β-catenin, β-catenin/E-cadherin, TGFβ/β-catenin and Met/β-catenin—in the context of prenatal liver development (Fig. 1). A general discussion of the role of β-catenin in normal liver growth and regeneration, as well as a general overview of embryonic liver development, will provide the background for this discussion.
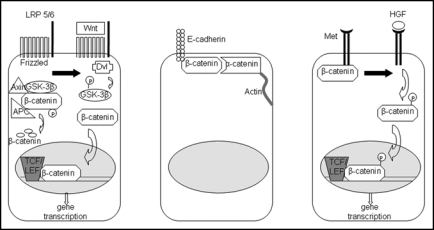
Figure 1.
The three major roles of β-catenin in liver physiology. Left: in the presence of Wnt, β-catenin is released from its inactivating complex and translocates to the nucleus, where it activates genes essential for proliferation, growth and regeneration of the liver. Middle: β-catenin mediates cell-cell adhesion through its interaction with e-cadherin on the hepatocyte membrane. Right: in the presence of HGF, β-catenin, which associates with Met at the surface of hepatocytes, is phosphorylated and translocates to the nucleus to turn on genes important in proliferation and morphogenesis.
Wnt/β-catenin Signaling in Normal Liver Growth and Regeneration
The Wnt/β-catenin pathway plays a key role in controlling post-natal liver growth. Our laboratory found an increase in β-catenin levels in wild-type mice shortly after birth,31 which serves to promote hepatic growth during postnatal development. Indeed, increased β-catenin translocation to the nucleus correlates with an increase in cell proliferation between 5–20 postnatal days.32 Further, mice expressing a conditional deletion of β-catenin generated by others and our laboratory showed a significant decrease in the liver weight/body weight ratio (15–25%) in mice older than two months.33,34 This decrease is correlated with basal decrease in cellular proliferation, which is due to a deficient cyclin-D1 response.
Several studies have addressed the role of constitutively active β-catenin on postnatal growth. Mice expressing an oncogenic form of β-catenin demonstrated hyperplasia and resulting hepatomegaly as young as 3–4 weeks after birth.35 Other reports have described hepatomegaly soon after birth in mouse strains where a dominant stable form of β-catenin was activated by adenoviral inoculation.36 Finally, we have recently generated transgenic mice overexpressing wild-type β-catenin and observed a 15% increase in liver size in these mice compared to normal wild-type aged matched controls.37 All three mouse models showed a correlation between increased nuclear localization of β-catenin and increased proliferation in the transgenic overexpressing mice, implicating activation of cell cycle regulators by β-catenin in the resulting phenotype. Notably, all of the β-catenin-overexpressing mouse models showed a distinct lack of spontaneous hepatic tumors, suggesting that β-catenin alone may be insufficient to cause tumorigenesis.
In adult resting liver, the Wnt/β-catenin pathway is quiescent. This steady-state condition is characterized by the phosphorylation and subsequent degradation that is the hallmark of β-catenin turnover; hence, β-catenin is localized at the cell membrane and is largely absent from the cytoplasm and nucleus.38 Therefore, when liver is not being challenged by chemical, metabolic or dietary stress, β-catenin is not required for normal physiologic function.34
However, during liver regeneration, levels of β-catenin are dramatically increased. The most common method used to study liver regeneration is the partial hepatectomy (PHx) model, in which two-thirds of the rat or mouse liver is removed; the remaining lobes enlarge to recapitulate the original liver mass.39 This model is an ideal environment to study the role of β-catenin in controlled growth after injury. In a rat model, an increase in β-catenin protein expression was seen as early as 1–5 minutes post-PHx;40 this increase was not due to an increase in mRNA expression, but rather to a decrease in protein degradation, the result of a change in steady-state kinetics. This expression was transient, and β-catenin levels returned to normal at 48 hours. Translocation of β-catenin to the nucleus, starting at 5 minutes after PHx and continuing until 48 hours post-PHx, contributes to the increase in cyclin D1 and c-myc and the concomitant increase in cellular proliferation.40
The importance of β-catenin to liver regeneration is highlighted by three studies in which β-catenin is removed or absent from the liver. When a β-catenin antisense oligonucleotide was administered to rats after 2/3 PHx, total β-catenin decreased significantly at 24 hours.41 Also of note was the decrease in liver weight/body weight ratio as a function of decreased proliferation in these animals. Further, the β-catenin knockout mice mentioned previously showed a sick and lethargic phenotype after PHx as opposed to their wild-type counterparts. Additionally, these mice displayed suboptimal regeneration, with delayed regenerative onset and a biphasic trend in proliferation that peaked at day 3 and increased slightly again at day 14.33 These results were confirmed by another laboratory that demonstrated a lack of cyclin D1 induction and a resultant delay in DNA synthesis in liver-specific β-catenin knockout mice.42 Interestingly, subjecting TOPGAL-reporter mice to partial hepatectomy revealed that the delay in proliferation occurred despite a lack of observed β-catenin activation; thus, the usefulness of this mouse model to the study of liver processes such as regeneration remains unclear.
The function of β-catenin in liver growth and regeneration emphasizes its vital role in the health and repair of adult liver. As fetal development is also a time of increased cellular growth in the liver, many of the same gene expression patterns seen in growth and regeneration are also present in prenatal liver organogenesis.43
Embryonic Liver Development
The induction of embryonic liver is a complex process that requires a series of tightly regulated localized signals from multiple cell types (Fig. 2). Liver in mouse begins to arise from the definitive gut endoderm at E8.5, or the 7–8 somite stage.44–46 It is at this time that a family of transcription factors, Foxa, specifies the endoderm to express hepatic genes.47,48 The fibroblast growth factors FGF1 and FGF2, which are expressed in the cardiac mesoderm at this time, are responsible for initiating the expression of these liver-specific genes in the endoderm.49 FGF8, which is important for morphogenetic outgrowth of the liver, is also expressed during this stage.49 Endothelial cells also interact with the hepatic endoderm shortly after specification to promote morphogenesis and liver bud formation.50 The resulting bud migrates into the septum transversum mesenchyme upon bone morphogenic protein 4 (BMP4) signaling, which is also required for hepatogenesis.51
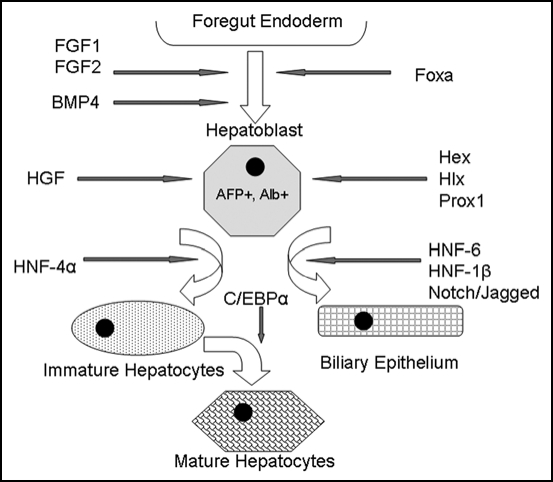
Figure 2.
Model of embryonic liver development. Signals such as FGF-1, FGF-2 and BMP4 emanating from the cardiac mesoderm specify the foregut endoderm to begin expressing liver-specific genes. The Foxa transcription factors are also required for foregut endoderm specification. The resulting hepatoblasts, which are albumin and a-fetoprotein positive, proliferate and expand. Hepatoblasts express transcription factors such as Hex, Hlx and Prox1, which are essential for liver proliferation and differentiation. HNF-6, HNF-1β and the Notch/Jagged signaling pathway induce differentiation toward a biliary epithelial lineage, while HNF-4α followed by C/EBPα produces mature hepatocytes.
The second phase of embryonic liver growth is characterized by expansion and proliferation. The cells are now considered hepatoblasts, which means that they are capable of giving rise to both major lineages of the liver, hepatocytes and biliary epithelial cells.52 HGF, expressed in the septum transversum mesenchyme which now surrounds the liver bud, is critical for this stage of liver growth.53 One of the most critical genes at this stage is Hex, which is maintained by FGF and BMP4 signaling in the commitment phase;54 expression of this transcription factor is essential for hepatoblast differentiation and liver bud expansion.55–57 Hepatoblasts or hepatic progenitors are the bipotential stem cells that will be undergoing expansion while maintaining their de-differentiated state during this stage. This event is comparable to the expansion of a lineage-restricted progenitor population in stem cell biology. Other transcription factors required for liver development at this stage include Hlx58 and Prox1.59 Albumin and a-fetoprotein mRNA is also being produced at this time, indicating commitment to a hepatic fate.60 Finally, the general architecture of the liver is beginning to be established, including the formation of sinusoids and the development of hepatic vasculature.47,61
The final stage is characterized by the differentiation of hepatoblasts to mature, fully-functional cell types. At the center of the differentiation process are the liver-enriched transcription factors such as hepatocyte nuclear factor (HNF) transcription factors and C/EBPα, which regulate cell fate decisions in the liver.62,63 HFN-4α is essential for differentiation toward a hepatocyte phenotype, as well as formation of the parenchyma;64 GATA6, which regulates HFN-4α, is also required for hepatocyte differentiation.65 HNF-6, HNF-1β and the Notch signaling pathway are required for normal development of biliary epithelia and resulting bile duct structures.66–68 Finally, the expression of transcription factors NFκB, c-Jun and XBP-1 is necessary for growth and morphogenesis,52 which continues until birth.
This somewhat simplistic outline of embryonic liver development is not meant to be comprehensive; we have emphasized factors such as FGF, BMP, Foxa and Hex that are either downstream targets or upstream regulators of the β-catenin pathway, while recognizing that there are many other pathways that play a role in liver embryogenesis. Nor does it take into account the complexity involved in the expression of these growth and transcription factors. Liver development is not a linear process; rather, there is significant overlap between gene expression patterns that blur the lines between one stage of liver development and the next. Additionally, activation of one gene may initiate a feedback mechanism that regulates cross-talk between different cell populations. For example, the HNFs are capable of auto-regulating their own expression as well as cross-regulating the transcription of other liver-specific genes.52 To complicate matters, a gene may be expressed in one zone and simultaneously repressed in a neighboring zone, which makes studying the timing of gene activation in the context of a specific region of the liver particularly important. Finally, the redundancy of the system in later stages of liver development means that other genes are often able to compensate for those that have been abolished. Nonetheless, the role of β-catenin in liver development, while still being elucidated, appears to be both a negative and a positive regulator at different times during development of the liver.69,70
The Role of Wnt/β-catenin in Embryonic Liver Development
The Wnt pathway is now recognized as one of the major regulators of embryonic development, controlling such processes as embryonic induction, polarity and cell fate specification.1 In C. elegans, mom genes, which are homologues of Wnt, establish polarity in the embryo by inducing endodermal cells to adopt a mesodermal fate.71 Depleting maternal β-catenin from Xenopus embryos results in reduced dorsal axial structures,72 resulting in lack of all dorsal-anterior mesoderm tissue. Even more strikingly, mice with targeted deletions of β-catenin display a block in anterior-posterior axis formation early in embryogenesis and subsequently fail to initiate gastrulation.73 Additionally, overexpression of Wnt8c in the mouse leads to duplication of axes.74 At the level of organ development, Wnt is important in epithelial-to-mesenchymal transition, as demonstrated by the inactivation of Wnt-4, which results in the absence of kidneys in a mouse embryo.75
In recent years, a plethora of evidence has emerged identifying regulation of Wnt/β-catenin signaling as a requirement for embryonic liver development as well. In fact, in Xenopus, the impact of Wnt/β-catenin signaling on liver development can be seen as early as the maternal phase, which occurs before gastrulation. Maternal Wnt/β-catenin, in conjunction with endodermally-derived TGFβ, can induce anterior endomesoderm (AE), a subset of endoderm cells fated to form the liver.76 Thereafter, repression of this pathway becomes necessary during the competency and commitment stage of liver development.77 In Xenopus, β-catenin expression after gastrulation is necessary for intestinal formation in the posterior endoderm, while repression in the anterior endoderm allows for expression of Hex, which is required for liver and pancreas development. Repressing β-catenin in the posterior endoderm causes organ buds expressing liver markers to form.78
Several other studies have found that inhibitors of the Wnt signaling pathway can influence early liver formation. Sfrp5, an antagonist of Wnt, is expressed in the ventral foregut endoderm that gives rise to the liver at mouse E8.5,79 resembling the expression pattern of Hex. The expression of this inhibitor may function to modulate Wnt activity by delineating borders between organs in the developing gut.80 Further, calcineurin, a member of the Wnt/calcium signaling pathway, is involved in dorsal-side signaling that leads to the formation of liver during Xenopus embryogenesis through its interference with canonical Wnt/β-catenin signaling.81
Interestingly, a recent study proposes just the opposite: that β-catenin expression is required during the liver specification stage.82 A conditional mutant of prometheus, a homologue of Wnt2b in zebrafish, is expressed in the mesoderm directly adjacent to the developing liver; its deletion causes a severe but transient defect in liver formation. Further analysis revealed that expression of genes such as Hex and Prox1, which are essential in hepatoblast formation, is impaired in these mutants, thus implicating these transcription factors as potential downstream targets of the β-catenin pathway.82 This study, which suggests that the Wnt pathway is a positive regulator of liver specification, is a contradiction to the previous work demonstrating the necessity for β-catenin inhibition during the competency stage. One possible explanation could be that β-catenin is actually activated in the hepatic induction phase, which overlaps with the competence and specification stage. Another explanation, although an unlikely one, could be the difference in species. However, it is more likely that this discrepancy is more of a timing issue whereby initial repression of Wnt signaling is immediately followed by the activation of the pathway for liver outgrowth. Additional studies, perhaps using conditional knockout mice in which β-catenin is deleted before E8.5, will be needed to address this contradiction and definitively determine whether Wnt/β-catenin signaling is activated or repressed during liver specification.
Our laboratory was the first to demonstrate a mechanistic role for the Wnt/β-catenin pathway in developing liver. Livers from mouse embryos cultured in the presence of a β-catenin antisense oligonucleotide showed a decrease in proliferation and a simultaneous increase in apoptosis, two processes vital to liver development.83 This correlated well with a subsequent study that found overexpression of β-catenin in developing chicken livers leads to a three-fold increase in liver size, which is due at least in part to an expanded hepatoblast population. Conversely, blocking β-catenin expression through overexpression of pathway inhibitors resulted in decreased liver size and altered liver shape.84 The effect on cell proliferation noted in both cases may be due to cell cycle mediators such as cyclin D1, which is a known downstream target of β-catenin. Interestingly, an earlier study using a deletion of GSKβ demonstrated a phenotype of increased liver cell death and liver degeneration that resulted in embryonic lethality. Whether this effect was due to untimely β-catenin stabilization or due to the fact that GSK3β is at the crossroads of several other signaling pathways critical to liver biology, such as insulin signaling, remains to be investigated further.85 The concept of untimely β-catenin stabilization on liver growth and survival during development is also supported by a more recent study, which utilizes APC deletion during liver development. This study shows a dramatic increase in cell death and a counterintuitive decrease in cell proliferation.86 This clearly supports a highly temporal expression, activation and role of Wnt/β-catenin signaling during the process of normal liver development and any deviation from this norm would result in abnormal growth with consequences.
Indeed, we and others have shown that β-catenin protein expression peaks at E10–12, during which time it is localized throughout the cell including the nucleus, cytoplasm and membrane. Subsequent decreases in β-catenin gene expression and increased protein degradation coincide with a dramatic decrease in total β-catenin protein expression after E16, at which time it is also localized to the membrane of maturing hepatoblasts and hepatocytes.31,87 While the early stages coincide with ongoing hepatoblast expansion mediated via their increased proliferation and survival, the later stages represent hepatoblast maturation to hepatocytes that begin to express genes that are associates and measures of function such as transferring, cyochrome P450s, coagulation factors, haptoglobin and many others.88
As found in our previous studies with ex vivo embryonic liver cultures, there was also a positive correlation between β-catenin and cell proliferation, which has also been supported by additional studies in chicken, zebrafish and Xenopus.33,78,82–84 Thus, these studies have established an important physiological role for β-catenin during early liver development in expansion of hepatoblasts or the hepatic progenitors. Interestingly, the adult counterpart of this cell is the facultative stem cell, also known as the oval cell. Oval cells are also bipotential progenitors that are activated during regeneration when hepatocyte proliferation is impaired.89 These postnatal facultative hepatic progenitor cells have also been reported to be present in normal fetal livers.90 Consequently, it is interesting to note that Wnt signaling is important in the induction of an oval cell response. Mice fed a 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet, which enriches for oval cells, showed nuclear translocalization of β-catenin, which corresponded with an increase in proliferation, as expected.91 Similarly, we found an increase in active β-catenin in mice fed 2-acetylaminofluorine followed by partial hepatectomy, while β-catenin conditional knockout mice fed a DDC diet had a noticeable decrease in the number of oval cells.92 Since both normal embryonic liver development and oval cell activation after liver injury have this pathway in common, it appears that β-catenin is a shared link among many distinct physiological processes in the liver.
β-catenin also plays an important role in the differentiation of hepatoblasts into the two major liver lineage cell types: biliary epithelial cells and hepatocytes. The addition of Wnt3a to the explanted mouse embryos induced a biliary phenotype and duct-like arrangement in the developing liver, while lack of Wnt3a causes loss of architecture, proliferation and increased apoptosis.93 Interestingly, the β-catenin activation shown recently in the APC mutant mouse also imparts a pro-biliary differentiation to the hepatoblasts, clearly verifying our previous studies.86
We also observed a lack of mature hepatocytes in the absence of β-catenin in our ex vivo embryonic liver cultures. This phenotype was confirmed by the concomitant presence of stem cell markers and mature hepatocyte markers in the organ culture.83 Using another in vitro model that recapitulates hepatocyte differentiation, we found an increase in total β-catenin proteiβn as early as 24 hours after the induction of differentiation.94 This increase was a result of decreased protein degradation and resulted in membranous localization of β-catenin rather than the nuclear localization that is the hallmark of proliferating undifferentiated cells. Finally, deletion of β-catenin from hepatoblasts in vivo using β-catenin transgenic mice under the Foxa3 promoter resulted in the decrease of liver-specific transcription factors C/EBPα and HNF4α and an overall hepatic deficiency.95
These studies imply that the presence of β-catenin, as well as its location inside the cell, might be a critical event dictating differentiation. This is also supported by that fact that the Wnt/β-catenin pathway has recently been labeled as the zonation keeper of the liver.96 It regulates the expression of various genes that encode for proteins involved in ammonia metabolism, xenobiotic metabolism and others, all functions of differentiated hepatocytes.34,35,37 Whether some of these genes are direct transcriptional targets of β-catenin or are mediated by β-catenin's role in regulating some of the key liver-specific transcription factors remains to be elucidated.
Although the picture is far from complete, the data thus far suggests that β-catenin levels vary during prenatal hepatic development, and that the temporal expression of β-catenin regulates liver formation (Fig. 3). A major undetermined aspect of Wnt/β-catenin signaling during liver development remains the obscurity of upstream effectors such as Wnt/Fz genes and related proteins that are dictating the temporal expression and activation of β-catenin. However, spatiotemporal expression of β-catenin is not unique to the liver; the Wnt/β-catenin pathway also shows distinct stage specific effects during cardiac,97,98 gut,99 and lens development.100 For example, activating β-catenin expression during embryogenesis enhances cardiomyocyte differentiation, while activation of this pathway later in development causes inhibition of differentiation.101 In the gut, Wnt expression is present in developing intestines after the appearance of villi, disappears during villi morphogenesis, and then reappears in differentiated postmitotic villus epithelium.102 Thus, data from organogenesis studies demonstrates that turning β-catenin expression on and off at various stages of development can cause opposing results depending on the timing of induction.
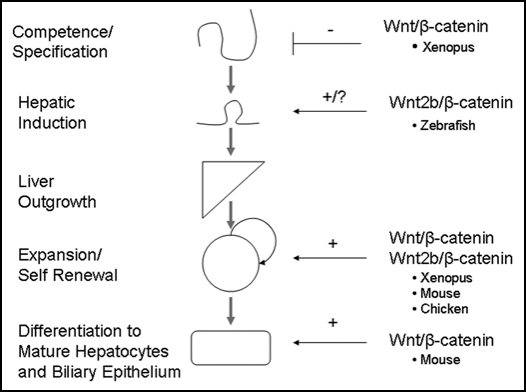
Figure 3.
Role of β-catenin in embryonic liver development. β-catenin expression must be suppressed during the competence and specification stage in order for normal liver development to occur. However, expression of β-catenin is essential during the later stages of liver development, such as outgrowth, expansion and differentiation. Although there is conflicting evidence concerning the role of β-catenin during very early liver development, we hypothesize that β-catenin expression is necessary for hepatic induction immediately after its repression during the competence/specification stage. Thus, β-catenin has been found to play a role in all stages of embryonic liver development.
The interaction of β-catenin with its other binding partners—Met and E-cadherin—can also impact liver development. We found increased association with Met during Matrigel-induced differentiation, which suggests that this interaction is crucial for hepatocyte maturation.94 The interaction of β-catenin with E-cadherin, which is important in regulating cell-cell adhesion, increases at E16 through E18.31 During this stage of liver development, β-catenin is principally located at the cell membrane. In fact, the ability to form intercellular adhesions, which is characterized by the Met/β-catenin and E-cadherin/β-catenin complexes, may be a marker of hepatic maturity.17
Studies into the mechanism of Wnt/β-catenin activation in liver development have implicated FGF proteins as upstream mediators of this pathway. Expression of FGF-10 in the mouse liver correlates with peak β-catenin activation; moreover, release of FGF-10 from stellate cells stimulates β-catenin expression in hepatoblasts.103 Our laboratory has found that FGF-2, FGF-4 and FGF-8 promote an increase in the number of hepatic progenitor cells in ex vivo embryonic livers. FGF-8 in particular seems to be crucial to the enrichment of progenitor cells as it promotes hepatocyte differentiation in addition to proliferation.104 Addition of these growth factors to explanted livers also induced β-catenin expression, suggesting that FGF activates the Wnt pathway. Since FGF-8 is also a downstream target of the Wnt pathway,105,106 these relationships are suggestive of a positive feedback mechanism in which β-catenin plays a central regulatory role (Fig. 4).
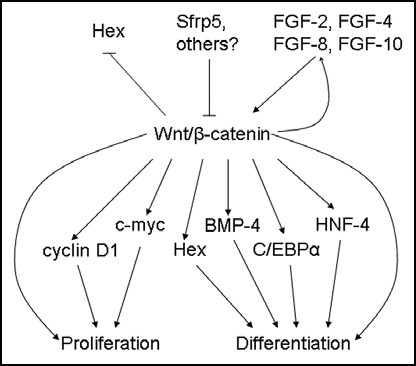
Figure 4.
Upstream regulators and downstream targets of β-catenin during embryonic liver development. β-catenin expression in early foregut development inhibits Hex expression; later in hepatogenesis, β-catenin is thought to regulate proliferation in part through Hex. Srfp5 inhibits β-catenin activity, while FGFs stimulate the β-catenin pathway. In turn, β-catenin activates FGF-8 expression, suggesting a feed-forward mechanism. Downstream targets of β-catenin, such as cyclin D1 and c-myc, induce proliferation, while others, such as Hex, BMP4, C/EBPα and HNF-4, promote differentiation.
Conclusions
In liver regeneration, the main function of the β-catenin pathway is to regulate cell proliferation. Data thus far suggests that β-catenin plays a similar role in liver development. As liver development is physiologically similar to liver regeneration, this conclusion would seem to be in agreement with previous studies and conclusively positions β-catenin as an integral part of liver biology.
A growing body of evidence points toward a crucial role of Wnt/β-catenin in liver development. Further studies will be necessary to identify upstream regulators of Wnt, as well as downstream targets of β-catenin, in order to better appreciate the impact of this pathway on developing liver. Tools such as conditional knockout mice, gene array analysis and embryonic liver cultures will continue to enhance our understanding of this important pathway.
Acknowlegdements
This review was supported in part by Rangos Fund for Enhancement of Pathology Research and by Grant support from National Institutes of Health-1R01DK62277 to S.P.S.M. and 1R01CA124414 to S.P.S.M.
Footnotes
Previously published online as an Organogenesis E-publication: http://www.landesbioscience.com/journals/organogenesis/article/5855
References
- 1.Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes & development. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 2.Nusse R. Wnt signaling in disease and in development. Cell research. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- 3.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 4.Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 5.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 6.Polakis P. Wnt signaling and cancer. Genes & development. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 7.Koch A, Denkhaus D, Albrecht S, Leuschner I, von Schweinitz D, Pietsch T. Childhood hepatoblastomas frequently carry a mutated degradation targeting box of the beta-catenin gene. Cancer Res. 1999;59:269–273. [PubMed] [Google Scholar]
- 8.Oda H, Imai Y, Nakatsuru Y, Hata J, Ishikawa T. Somatic mutations of the APC gene in sporadic hepatoblastomas. Cancer Res. 1996;56:3320–3323. [PubMed] [Google Scholar]
- 9.Miao J, Kusafuka T, Udatsu Y, Okada A. Sequence variants of the Axin gene in hepatoblastoma. Hepatol Res. 2003;25:174–179. doi: 10.1016/s1386-6346(02)00264-4. [DOI] [PubMed] [Google Scholar]
- 10.Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, Sasaki Y, Imaoka S, Murata M, Shimano T, Yamaoka Y, Nakamura Y. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Gen. 2000;24:245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- 11.de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A, Perret C. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci USA. 1998;95:8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong CM, Fan ST, Ng IO. beta-Catenin mutation and overexpression in hepatocellular carcinoma: clinicopathologic and prognostic significance. Cancer. 2001;92:136–145. doi: 10.1002/1097-0142(20010701)92:1<136::aid-cncr1301>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 13.Merle P, de la Monte S, Kim M, Herrmann M, Tanaka S, Von Dem Bussche A, Kew MC, Trepo C, Wands JR. Functional consequences of frizzled-7 receptor overexpression in human hepatocellular carcinoma. Gastroenterology. 2004;127:1110–1122. doi: 10.1053/j.gastro.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Ban KC, Singh H, Krishnan R, Seow HF. GSK-3beta phosphorylation and alteration of beta-catenin in hepatocellular carcinoma. Cancer Lett. 2003;199:201–208. doi: 10.1016/s0304-3835(03)00421-x. [DOI] [PubMed] [Google Scholar]
- 15.Behari J, Zeng G, Otruba W, Thompson MD, Muller P, Micsenyi A, Sekhon SS, Leoni L, Monga SP. R-Etodolac decreases beta-catenin levels along with survival and proliferation of hepatoma cells. J Hepatol. 2007;46:849–857. doi: 10.1016/j.jhep.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng G, Apte U, Cieply B, Singh S, Monga SP. siRNA-mediated beta-catenin knockdown in human hepatoma cells results in decreased growth and survival. Neoplasia. 2007;9:951–959. doi: 10.1593/neo.07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology. 2007;45:1298–1305. doi: 10.1002/hep.21651. [DOI] [PubMed] [Google Scholar]
- 18.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. J Cell Sci. 2006;119:395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- 20.Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin. Curr Opin Cell Biol. 2005;17:459–465. doi: 10.1016/j.ceb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem. 1999;274:36734–36740. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- 22.Theard D, Steiner M, Kalicharan D, Hoekstra D, van Ijzendoorn SC. Cell polarity development and protein trafficking in hepatocytes lacking E-cadherin/beta-catenin-based adherens junctions. Mol Biol Cell. 2007;18:2313–2321. doi: 10.1091/mbc.E06-11-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang Y, Kitisin K, Jogunoori W, Li C, Deng CX, Mueller S, Ressom H, Rashid A, He AR, Mendelson JS, Shetty K, Zasloff M, Mishra B, Reddy EP, Johnson L, Mishra L. Progenitor/stem cells give rise to liver cancer due to aberrant TGF-beta and IL-6 signaling. Proc Natl Acad Sci USA. 2008 doi: 10.1073/pnas.0705395105. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peinado H, Quintanilla M, Cano A. Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J Biol Chem. 2003;278:21113–21123. doi: 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
- 25.Katuri V, Tang Y, Li C, Jogunoori W, Deng CX, Rashid A, Sidawy AN, Evans S, Reddy EP, Mishra B, Mishra L. Critical interactions between TGF-beta signaling/ELF, and E-cadherin/beta-catenin mediated tumor suppression. Oncogene. 2006;25:1871–1886. doi: 10.1038/sj.onc.1209211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zarnegar R. Regulation of HGF and HGFR gene expression. Exs. 1995;74:33–49. doi: 10.1007/978-3-0348-9070-0_3. [DOI] [PubMed] [Google Scholar]
- 27.Monga SP, Mars WM, Pediaditakis P, Bell A, Mule K, Bowen WC, Wang X, Zarnegar R, Michalopoulos GK. Hepatocyte growth factor induces Wnt-independent nuclear translocation of beta-catenin after Met-beta-catenin dissociation in hepatocytes. Cancer Res. 2002;62:2064–2071. [PubMed] [Google Scholar]
- 28.Apte U, Zeng G, Muller P, Tan X, Micsenyi A, Cieply B, Dai C, Liu Y, Kaestner KH, Monga SP. Activation of Wnt/beta-catenin pathway during hepatocyte growth factor-induced hepatomegaly in mice. Hepatology. 2006;44:992–1002. doi: 10.1002/hep.21317. [DOI] [PubMed] [Google Scholar]
- 29.Ishibe S, Haydu JE, Togawa A, Marlier A, Cantley LG. Cell confluence regulates hepatocyte growth factor-stimulated cell morphogenesis in a beta-catenin-dependent manner. Mol Cell Biol. 2006;26:9232–9243. doi: 10.1128/MCB.01312-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tward AD, Jones KD, Yant S, Cheung ST, Fan ST, Chen X, Kay MA, Wang R, Bishop JM. Distinct pathways of genomic progression to benign and malignant tumors of the liver. Proc Natl Acad Sci USA. 2007;104:14771–14776. doi: 10.1073/pnas.0706578104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Micsenyi A, Tan X, Sneddon T, Luo JH, Michalopoulos GK, Monga SP. Beta-catenin is temporally regulated during normal liver development. Gastroenterology. 2004;126:1134–1146. doi: 10.1053/j.gastro.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 32.Apte U, Zeng G, Thompson MD, Muller P, Micsenyi A, Cieply B, Kaestner KH, Monga SP. beta-Catenin is critical for early postnatal liver growth. Am J Physiol. 2007;292:1578–1585. doi: 10.1152/ajpgi.00359.2006. [DOI] [PubMed] [Google Scholar]
- 33.Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131:1561–1572. doi: 10.1053/j.gastro.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 34.Sekine S, Lan BY, Bedolli M, Feng S, Hebrok M. Liver-specific loss of beta-catenin blocks glutamine synthesis pathway activity and cytochrome p450 expression in mice. Hepatology. 2006;43:817–825. doi: 10.1002/hep.21131. [DOI] [PubMed] [Google Scholar]
- 35.Cadoret A, Ovejero C, Saadi-Kheddouci S, Souil E, Fabre M, Romagnolo B, Kahn A, Perret C. Hepatomegaly in transgenic mice expressing an oncogenic form of beta-catenin. Cancer Res. 2001;61:3245–3249. [PubMed] [Google Scholar]
- 36.Harada N, Miyoshi H, Murai N, Oshima H, Tamai Y, Oshima M, Taketo MM. Lack of tumorigenesis in the mouse liver after adenovirus-mediated expression of a dominant stable mutant of beta-catenin. Cancer Res. 2002;62:1971–1977. [PubMed] [Google Scholar]
- 37.Tan X, Apte U, Micsenyi A, Kotsagrelos E, Luo JH, Ranganathan S, Monga DK, Bell A, Michalopoulos GK, Monga SP. Epidermal growth factor receptor: a novel target of the Wnt/beta-catenin pathway in liver. Gastroenterology. 2005;129:285–302. doi: 10.1053/j.gastro.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez FJ. Role of beta-catenin in the adult liver. Hepatology. 2006;43:650–653. doi: 10.1002/hep.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 40.Monga SP, Pediaditakis P, Mule K, Stolz DB, Michalopoulos GK. Changes in WNT/beta-catenin pathway during regulated growth in rat liver regeneration. Hepatology. 2001;33:1098–1109. doi: 10.1053/jhep.2001.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sodhi D, Micsenyi A, Bowen WC, Monga DK, Talavera JC, Monga SP. Morpholino oligonucleotide-triggered beta-catenin knockdown compromises normal liver regeneration. Journal of hepatology. 2005;43:132–141. doi: 10.1016/j.jhep.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Sekine S, Gutierrez PJ, Lan BY, Feng S, Hebrok M. Liver-specific loss of beta-catenin results in delayed hepatocyte proliferation after partial hepatectomy. Hepatology. 2007;45:361–368. doi: 10.1002/hep.21523. [DOI] [PubMed] [Google Scholar]
- 43.Haber B, Naji L, Cressman D, Taub R. Coexpression of liver-specific and growth-induced genes in perinatal and regenerating liver: attainment and maintenance of the differentiated state during rapid proliferation. Hepatology. 1995;22:906–914. [PubMed] [Google Scholar]
- 44.Zaret KS. Molecular genetics of early liver development. Ann. Rev Physiol. 1996;58:231–251. doi: 10.1146/annurev.ph.58.030196.001311. [DOI] [PubMed] [Google Scholar]
- 45.Zaret KS. Hepatocyte differentiation: from the endoderm and beyond. Curr Opin Genet Dev. 2001;11:568–574. doi: 10.1016/s0959-437x(00)00234-3. [DOI] [PubMed] [Google Scholar]
- 46.Zaret KS. Regulatory phases of early liver development: paradigms of organogenesis. Nat Rev. 2002;3:499–512. doi: 10.1038/nrg837. [DOI] [PubMed] [Google Scholar]
- 47.Spear BT, Jin L, Ramasamy S, Dobierzewska A. Transcriptional control in the mammalian liver: liver development, perinatal repression, and zonal gene regulation. Cell Mol Life Sci. 2006;63:2922–2938. doi: 10.1007/s00018-006-6258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee CS, Friedman JR, Fulmer JT, Kaestner KH. The initiation of liver development is dependent on Foxa transcription factors. Nature. 2005;435:944–947. doi: 10.1038/nature03649. [DOI] [PubMed] [Google Scholar]
- 49.Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284:1998–2003. doi: 10.1126/science.284.5422.1998. [DOI] [PubMed] [Google Scholar]
- 50.Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563. doi: 10.1126/science.1063889. [DOI] [PubMed] [Google Scholar]
- 51.Rossi JM, Dunn NR, Hogan BL, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15:1998–2009. doi: 10.1101/gad.904601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duncan SA. Transcriptional regulation of liver development. Dev Dyn. 2000;219:131–142. doi: 10.1002/1097-0177(2000)9999:9999<::aid-dvdy1051>3.3.co;2-e. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 54.Zhang W, Yatskievych TA, Baker RK, Antin PB. Regulation of Hex gene expression and initial stages of avian hepatogenesis by Bmp and Fgf signaling. Dev Biol. 2004;268:312–326. doi: 10.1016/j.ydbio.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 55.Martinez Barbera JP, Clements M, Thomas P, Rodriguez T, Meloy D, Kioussis D, Beddington RS. The homeobox gene Hex is required in definitive endodermal tissues for normal forebrain, liver and thyroid formation. Development. 2000;127:2433–2445. doi: 10.1242/dev.127.11.2433. [DOI] [PubMed] [Google Scholar]
- 56.Bort R, Signore M, Tremblay K, Martinez Barbera JP, Zaret KS. Hex homeobox gene controls the transition of the endoderm to a pseudostratified, cell emergent epithelium for liver bud development. Dev Biol. 2006;290:44–56. doi: 10.1016/j.ydbio.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Hunter MP, Wilson CM, Jiang X, Cong R, Vasavada H, Kaestner KH, Bogue CW. The homeobox gene Hhex is essential for proper hepatoblast differentiation and bile duct morphogenesis. Dev Biol. 2007;308:355–367. doi: 10.1016/j.ydbio.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hentsch B, Lyons I, Li R, Hartley L, Lints TJ, Adams JM, Harvey RP. Hlx homeo box gene is essential for an inductive tissue interaction that drives expansion of embryonic liver and gut. Genes Dev. 1996;10:70–79. doi: 10.1101/gad.10.1.70. [DOI] [PubMed] [Google Scholar]
- 59.Sosa-Pineda B, Wigle JT, Oliver G. Hepatocyte migration during liver development requires Prox1. Nat Gen. 2000;25:254–255. doi: 10.1038/76996. [DOI] [PubMed] [Google Scholar]
- 60.Zaret K. Early liver differentiation: genetic potentiation and multilevel growth control. Current opinion in genetics & development. 1998;8:526–531. doi: 10.1016/s0959-437x(98)80006-3. [DOI] [PubMed] [Google Scholar]
- 61.Zaret KS. Liver specification and early morphogenesis. Mech Dev. 2000;92:83–88. doi: 10.1016/s0925-4773(99)00326-3. [DOI] [PubMed] [Google Scholar]
- 62.Odom DT, Zizlsperger N, Gordon DB, Bell GW, Rinaldi NJ, Murray HL, Volkert TL, Schreiber J, Rolfe PA, Gifford DK, Fraenkel E, Bell GI, Young RA. Control of pancreas and liver gene expression by HNF transcription factors. Science. 2004;303:1378–1381. doi: 10.1126/science.1089769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Darlington GJ, Wang N, Hanson RW. C/EBP alpha: a critical regulator of genes governing integrative metabolic processes. Curr Opin Genet Dev. 1995;5:565–570. doi: 10.1016/0959-437x(95)80024-7. [DOI] [PubMed] [Google Scholar]
- 64.Parviz F, Matullo C, Garrison WD, Savatski L, Adamson JW, Ning G, Kaestner KH, Rossi JM, Zaret KS, Duncan SA. Hepatocyte nuclear factor 4alpha controls the development of a hepatic epithelium and liver morphogenesis. Nat Gen. 2003;34:292–296. doi: 10.1038/ng1175. [DOI] [PubMed] [Google Scholar]
- 65.Zhao R, Watt AJ, Li J, Luebke-Wheeler J, Morrisey EE, Duncan SA. GATA6 is essential for embryonic development of the liver but dispensable for early heart formation. Mol Cell Biol. 2005;25:2622–2631. doi: 10.1128/MCB.25.7.2622-2631.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clotman F, Lannoy VJ, Reber M, Cereghini S, Cassiman D, Jacquemin P, Roskams T, Rousseau GG, Lemaigre FP. The onecut transcription factor HNF6 is required for normal development of the biliary tract. Development. 2002;129:1819–1828. doi: 10.1242/dev.129.8.1819. [DOI] [PubMed] [Google Scholar]
- 67.Coffinier C, Gresh L, Fiette L, Tronche F, Schutz G, Babinet C, Pontoglio M, Yaniv M, Barra J. Bile system morphogenesis defects and liver dysfunction upon targeted deletion of HNF1beta. Development. 2002;129:1829–1838. doi: 10.1242/dev.129.8.1829. [DOI] [PubMed] [Google Scholar]
- 68.Lorent K, Yeo SY, Oda T, Chandrasekharappa S, Chitnis A, Matthews RP, Pack M. Inhibition of Jagged-mediated Notch signaling disrupts zebrafish biliary development and generates multi-organ defects compatible with an Alagille syndrome phenocopy. Development. 2004;131:5753–5766. doi: 10.1242/dev.01411. [DOI] [PubMed] [Google Scholar]
- 69.Burke ZD, Thowfeequ S, Tosh D. Liver specification: a new role for Wnts in liver development. Curr Biol. 2006;16:688–690. doi: 10.1016/j.cub.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 70.Shackel N. Zebrafish and the understanding of liver development: the emerging role of the Wnt pathway in liver biology. Hepatology. 2007;45:540–541. doi: 10.1002/hep.21543. [DOI] [PubMed] [Google Scholar]
- 71.Thorpe CJ, Schlesinger A, Carter JC, Bowerman B. Wnt signaling polarizes an early C. elegans blastomere to distinguish endoderm from mesoderm. Cell. 1997;90:695–705. doi: 10.1016/s0092-8674(00)80530-9. [DOI] [PubMed] [Google Scholar]
- 72.Heasman J, Crawford A, Goldstone K, Garner Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C. Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 73.Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121:3529–3537. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- 74.Popperl H, Schmidt C, Wilson V, Hume CR, Dodd J, Krumlauf R, Beddington RS. Misexpression of Cwnt8C in the mouse induces an ectopic embryonic axis and causes a truncation of the anterior neuroectoderm. Development. 1997;124:2997–3005. doi: 10.1242/dev.124.15.2997. [DOI] [PubMed] [Google Scholar]
- 75.Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- 76.Zorn AM, Butler K, Gurdon JB. Anterior endomesoderm specification in Xenopus by Wnt/beta-catenin and TGFbeta signalling pathways. Developmental biology. 1999;209:282–297. doi: 10.1006/dbio.1999.9257. [DOI] [PubMed] [Google Scholar]
- 77.Lemaigre F, Zaret KS. Liver development update: new embryo models, cell lineage control, and morphogenesis. Curr Opin Genet Dev. 2004;14:582–590. doi: 10.1016/j.gde.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 78.McLin VA, Rankin SA, Zorn AM. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207–2217. doi: 10.1242/dev.001230. [DOI] [PubMed] [Google Scholar]
- 79.Finley KR, Tennessen J, Shawlot W. The mouse secreted frizzled-related protein 5 gene is expressed in the anterior visceral endoderm and foregut endoderm during early post-implantation development. Gene Expr Patterns. 2003;3:681–684. doi: 10.1016/s1567-133x(03)00091-7. [DOI] [PubMed] [Google Scholar]
- 80.Pilcher KE, Krieg PA. Expression of the Wnt inhibitor, sFRP5, in the gut endoderm of Xenopus. Gene Expr Patterns. 2002;2:369–372. doi: 10.1016/s1567-133x(02)00023-6. [DOI] [PubMed] [Google Scholar]
- 81.Yoshida Y, Kim S, Chiba K, Kawai S, Tachikawa H, Takahashi N. Calcineurin inhibitors block dorsal-side signaling that affect late-stage development of the heart, kidney, liver, gut and somitic tissue during Xenopus embryogenesis. Dev Growth Differ. 2004;46:139–152. doi: 10.1111/j.1440-169X.2004.00733.x. [DOI] [PubMed] [Google Scholar]
- 82.Ober EA, Verkade H, Field HA, Stainier DY. Mesodermal Wnt2b signalling positively regulates liver specification. Nature. 2006;442:688–691. doi: 10.1038/nature04888. [DOI] [PubMed] [Google Scholar]
- 83.Monga SP, Monga HK, Tan X, Mule K, Pediaditakis P, Michalopoulos GK. Beta-catenin antisense studies in embryonic liver cultures: role in proliferation, apoptosis, and lineage specification. Gastroenterology. 2003;124:202–216. doi: 10.1053/gast.2003.50000. [DOI] [PubMed] [Google Scholar]
- 84.Suksaweang S, Lin CM, Jiang TX, Hughes MW, Widelitz RB, Chuong CM. Morphogenesis of chicken liver: identification of localized growth zones and the role of beta-catenin/Wnt in size regulation. Dev Biol. 2004;266:109–122. doi: 10.1016/j.ydbio.2003.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NFkappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 86.Decaens T, Godard C, de Reynies A, Rickman DS, Tronche F, Couty JP, Perret C, Colnot S. Stabilization of beta-catenin affects mouse embryonic liver growth and hepatoblast fate. Hepatology. 2007 doi: 10.1002/hep.21952. [DOI] [PubMed] [Google Scholar]
- 87.Wang QM, Yang KM, Zhou HY, Li X, Yang HJ, Li YS. The role of beta-catenin in rat embryonic development and tumorigenesis. Sichuan Da Xue Xue Bao Yi Xue Ban. 2006;37:872–875. [PubMed] [Google Scholar]
- 88.Monga SP, Hout MS, Baun MJ, Micsenyi A, Muller P, Tummalapalli L, Ranade AR, Luo JH, Strom SC, Gerlach JC. Mouse fetal liver cells in artificial capillary beds in three-dimensional four-compartment bioreactors. Am J Pathol. 2005;167:1279–1292. doi: 10.1016/S0002-9440(10)61215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fausto N, Campbell JS. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mec Dev. 2003;120:117–130. doi: 10.1016/s0925-4773(02)00338-6. [DOI] [PubMed] [Google Scholar]
- 90.Masson NM, Currie IS, Terrace JD, Garden OJ, Parks RW, Ross JA. Hepatic progenitor cells in human fetal liver express the oval cell marker Thy-1. Am J Physiol. 2006;291:45–54. doi: 10.1152/ajpgi.00465.2005. [DOI] [PubMed] [Google Scholar]
- 91.Hu M, Kurobe M, Jeong YJ, Fuerer C, Ghole S, Nusse R, Sylvester KG. Wnt/beta-catenin signaling in murine hepatic transit amplifying progenitor cells. Gastroenterology. 2007;133:1579–1591. doi: 10.1053/j.gastro.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 92.Apte U, Thompson MD, Cui S, Liu B, Cieply B, Monga SP. Wnt/beta-catenin signaling mediates oval cell response in rodents. Hepatology. 2007 doi: 10.1002/hep.21973. [DOI] [PubMed] [Google Scholar]
- 93.Hussain SZ, Sneddon T, Tan X, Micsenyi A, Michalopoulos GK, Monga SP. Wnt impacts growth and differentiation in ex vivo liver development. Exp Cell Res. 2004;292:157–169. doi: 10.1016/j.yexcr.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 94.Monga SP, Micsenyi A, Germinaro M, Apte U, Bell A. beta-Catenin regulation during matrigel-induced rat hepatocyte differentiation. Cell Tissue Res. 2006;323:71–79. doi: 10.1007/s00441-005-0045-8. [DOI] [PubMed] [Google Scholar]
- 95.Tan X, Yuan Y, Zeng G U A, Thompson MD, Cieply B, Stolz DB, Michalopoulos GK, Kaestner KH, Monga SP. Beta-catenin deletion in hepatoblasts disrupts hepatic morphogenesis and survival during mouse development. Hepatology. 2008;47:1–13. doi: 10.1002/hep.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Burke ZD, Tosh D. The Wnt/beta-catenin pathway: master regulator of liver zonation? Bioessays. 2006;28:1072–1077. doi: 10.1002/bies.20485. [DOI] [PubMed] [Google Scholar]
- 97.Eisenberg LM, Eisenberg CA. Evaluating the role of Wnt signal transduction in promoting the development of the heart. The Scientific World Journal. 2007;7:161–176. doi: 10.1100/tsw.2007.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tzahor E. Wnt/beta-catenin signaling and cardiogenesis: timing does matter. Dev Cell. 2007;13:10–13. doi: 10.1016/j.devcel.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 99.Lickert H, Kispert A, Kutsch S, Kemler R. Expression patterns of Wnt genes in mouse gut development. Mec Dev. 2001;105:181–184. doi: 10.1016/s0925-4773(01)00390-2. [DOI] [PubMed] [Google Scholar]
- 100.Ang SJ, Stump RJ, Lovicu FJ, McAvoy JW. Spatial and temporal expression of Wnt and Dickkopf genes during murine lens development. Gene Expr Patterns. 2004;4:289–295. doi: 10.1016/j.modgep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 101.Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, Komuro I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci USA. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim BM, Mao J, Taketo MM, Shivdasani RA. Phases of canonical Wnt signaling during the development of mouse intestinal epithelium. Gastroenterology. 2007;133:529–538. doi: 10.1053/j.gastro.2007.04.072. [DOI] [PubMed] [Google Scholar]
- 103.Berg T, Rountree CB, Lee L, Estrada J, Sala FG, Choe A, Veltmaat JM, De Langhe S, Lee R, Tsukamoto H, Crooks GM, Bellusci S, Wang KS. Fibroblast growth factor 10 is critical for liver growth during embryogenesis and controls hepatoblast survival via beta-catenin activation. Hepatology. 2007;46:1187–1197. doi: 10.1002/hep.21814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sekhon SS, Tan X, Micsenyi A, Bowen WC, Monga SP. Fibroblast growth factor enriches the embryonic liver cultures for hepatic progenitors. Am J Pathol. 2004;164:2229–2240. doi: 10.1016/S0002-9440(10)63779-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kawakami Y, Capdevila J, Buscher D, Itoh T, Rodriguez Esteban C, Izpisua Belmonte JC. WNT signals control FGF-dependent limb initiation and AER induction in the chick embryo. Cell. 2001;104:891–900. doi: 10.1016/s0092-8674(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 106.Shu W, Guttentag S, Wang Z, Andl T, Ballard P, Lu MM, Piccolo S, Birchmeier W, Whitsett JA, Millar SE, Morrisey EE. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev Biol. 2005;283:226–239. doi: 10.1016/j.ydbio.2005.04.014. [DOI] [PubMed] [Google Scholar]