Abstract
Wnt signals play a critical role in regulating the normal development of the mammary gland and dysregulation of Wnt signaling causes breast cancer. This pathway is involved in the earliest development of the mammary gland in embryos and its role extends through the functional differentiation of the gland during pregnancy. In this review, we summarize the molecular mechanisms through which Wnts regulate mammary gland development in the mouse.
Key words: Wnt, mammary gland, embryo, postnatal, cancer, stem cell
Introduction
The Wnt signaling pathway has long been recognized as having important roles in development and cancer. Wnts regulate a variety of cellular activities, including cell fate determination, proliferation, migration, polarity and gene expression.1 Many Wnts are essential during embryogenesis and they are also active in regenerating adult tissues, such as colon, skin, hair follicles, lymphoid tissues and bone.2–4 In all these sites, Wnt signaling appears to be critical for maintaining a proper balance between proliferation and differentiation. Not surprisingly then, Wnts have also been implicated in tumor formation in several organs.5,6 There are at least nineteen different Wnt genes, ten Frizzled (Fzd) receptor genes and two Lrp (low density lipoprotein (LDL) receptor-related protein) co-receptor genes within the mammalian genome. This large cast of characters provides for much potential diversity of signaling, which is further amplified by crosstalk with other growth factor signaling pathways at multiple levels. Furthermore, Wnt genes show temporally restricted and highly localized expression patterns, allowing individual Wnt family members to have specific and non-redundant functions at sites in which their expression overlaps, or to exert similar functions at different sites or times when they are expressed in a non-overlapping fashion. As we will discuss, Wnt signaling is necessary for the formation of a functioning mammary gland. However, our understanding of the full details of the functions of individual Wnts during mammary gland development is limited. In this review, we will first describe the Wnt signaling pathway and the development of the mammary gland from embryogenesis to pregnancy. Then we will outline what we know about the contribution of Wnt signaling and individual Wnts to each stage of mouse mammary gland development.
Wnt Signaling
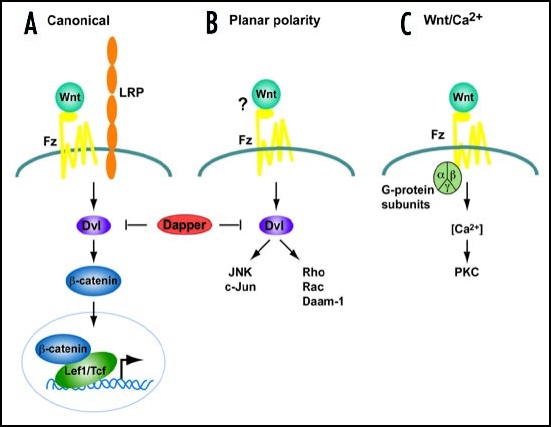
Wnts are secreted, lipid-modified glycoproteins that activate several different cell surface receptor-mediated signal transduction pathways. Wnt signaling cascades can be broadly subdivided into two categories, the canonical β-catenin pathway and the non-canonical pathway. Traditionally it has been thought that Wnt signals are transduced through the canonical pathway in order to regulate cell fate determination, and through the non-canonical pathway in order to control cell movement and tissue polarity (Fig. 1). In addition, some noncanonical Wnts can directly antagonize canonical signaling.7–9 The canonical or Wnt/β-catenin pathway (Fig. 2), promotes cell fate determination, proliferation and survival by increasing β-catenin levels and altering gene expression through lymphoid enhancer factor/T cell factor (Lef/Tcf) transcription factors.10 Free β-catenin in the cytoplasm is normally phosphorylated and degraded. Activation of canonical Wnt signaling inhibits β-catenin degradation, allowing it to accumulate in the cytoplasm and enter the nucleus, where it interacts with Lef/Tcfs in order to regulate gene expression. Wnt proteins released from or presented on the surface of signaling cells act on target cells by binding to cell surface receptor complexes. Canonical signaling relies on the interaction of Wnts with a co-receptor complex consisting of one of ten different Frizzled (Fzd) proteins and one of two low density lipoprotein (LDL) receptor-related proteins, Lrp5 or 6. Frizzleds are a sub-class of seven transmembrane-spanning G protein-coupled receptors, which participate in both canonical and non-canonical pathways.11,12 Lrp5 and Lrp6, are single-pass transmembrane proteins that appear to be specifically involved in the canonical pathway.13–16 When Wnts bind to this receptor complex, the PDZ-containing protein dishevelled (Dvl) is recruited to interact with Fzd, and another scaffolding protein, Axin, is recruited to bind Lrp5/6.17,18 Recruitment of Dvl and Axin disrupts a multiprotein complex containing glycogen synthase kinase 3β (GSK3β), which normally phosphorylates β-catenin and leads to its ubiquination and degradation. Inhibition of GSKβ, thus allows β-catenin molecules to accumulate in the cytoplasm and enter the nucleus to affect gene expression.10
Figure 1.
Simplified schemes of the three Wnt signaling cascades. (A) Canonical Pathway. A Wnt ligand binds to a Frizzled receptor and a co-receptor of low density lipoprotein (Lrp) family. Dishevelled (Dvl) and β-catenin are required to transduce the Wnt signal, leading to a transcriptional response mediated by transcription factors of the Tcf/Lef1 family. (B) Planar cell Polarity pathway. In this pathway, Dvl also transduces signals although independent of β-catenin activating JNK and Rho kinase cascades. (C) The Wnt/Ca2+ pathway signals via heterotrimeric G-proteins to mobilize intracellular Ca2+ and stimulate protein kinase C (PKC). The requirement for Dvl is controversial. In vertebrates this pathway is activated by the same ligands as (B). Dapper, a Dvl-associated antogonist, antagonizes both (A and B) pathways.
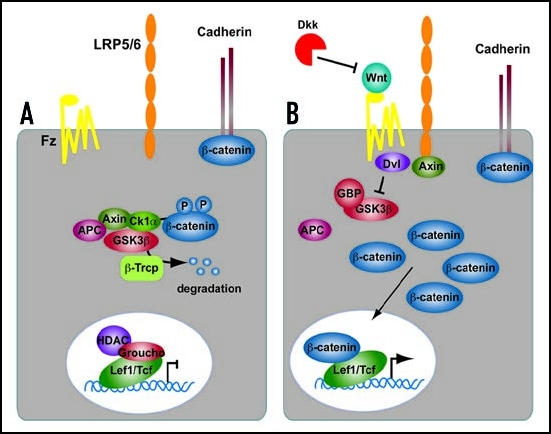
Figure 2.
Summary of the Canonical Wnt signaling cascade. (A) In the absence of a Wnt ligand, β-catenin is degraded through interactions between APC, Axin and the protein kinase GSK3β. In the nucleus, Lef1/Tcf transcription factors are in complexes with corepressors (e.g., CtBP, HDAC, Groucho) thereby repressing the transcription of Wnt target genes. (B) Wnt proteins bind to the Frizzled/Lrp receptor complex at the cell surface. These receptors transduce a signal to Dishevelled (Dvl) and to Axin, which may directly interact. As a consequence, β-catenin degradation is inhibited, and it accumulates in the cytoplasm and nucleus. β-catenin interacts with Lef1/Tcfs in the nucleus to control transcription of Wnt target genes. The secreted antagonist Dickkopf (Dkk) does not bind to Wnt ligands directly but prevents formation of an active Wnt-Fzd-Lrp complex.
Once in the nucleus, β-catenin displaces transcriptional co-repressors such as CtBP and Groucho19–22 and histone deacetylases23,24 from Lef1/Tcfs and directly binds to the amino-terminus of Lef1/Tcfs. Transcriptional co-activators such as CBP/p300, Bcl9 and Pygopus25–29 are subsequently recruited to the complex, which then is able to activate the expression of many genes.24,25,30–32 Some genes are repressed by Wnt signaling. Naturally occurring dominant negative forms of Lef1/Tcf proteins inhibit β-catenin dependent transcription in the nucleus.33 Canonical Wnt signaling also inhibits gene expression by less understood mechanisms that may depend on the availability of other proteins.34–37 An updated list of genes affected by Wnt signaling can be found on the Wnt Gene Homepage (http://www.stanford.edu/rnusse/Wntwindow.html). In addition to factors regulating the Wnt signalling cascade intracellularly, several extracellular proteins also negatively regulate canonical Wnt signaling. Dickkopfs (Dkks) and secreted frizzled related proteins (Sfrps) are two families of extracellular factors that antagonize Wnt activities.38,39 Dkks limit the availability of Lrp5/6 receptors to Wnts by sequestering Lrp5/6 into complexes with Kremens (Krm) and possibly promoting their internalization to lysosomes.16,17,40,41 In contrast, Sfrps bind directly to Wnts and prevent their association with Lrp and Fzd receptors on the cell surface.42
Relatively less is know about the non-canonical Wnt signaling pathways, although there is growing interest in these pathways. Non-canonical Wnt signals are transduced through Fzd family receptors and co-receptors independent of GSK3β and β-catenin. The planar polarity pathway activates Rho/Rac GTPases and Jun N-terminal kinase to modulate cytoskeletal organization and gene expression.43 The Wnt/Ca2+ pathway, stimulates heterotrimeric G proteins, increases intracellular calcium levels, decreases cyclic GMP levels, and activates protein kinase C to activate NFAT and other transcription factors.44 Distinct Wnt ligands act through specific Frizzled (Fzd) receptors to initiate each pathway.45,46
Overview of Mammary Gland Development
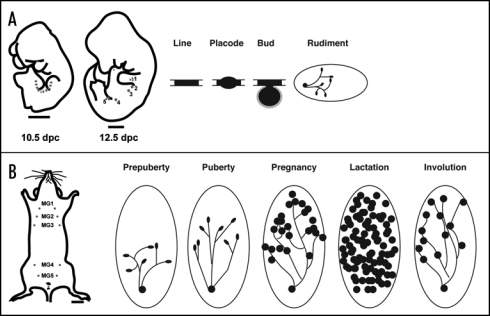
Traditionally, mammary development has been divided into several stages during which specific developmental/physiological tasks are completed. These stages span both embryonic and postnatal life because while development of the gland begins in embryos, it is only completed in adults during pregnancy (reviewed in ref. 47, Fig. 3). The earliest sign of mammary glands occurs in mice at around embryonic day 10, when the mammary lines develop between the limb buds. These are thickenings of the fetal epidermis which can be defined both morphologically as well as through the use of specific molecular markers. Between E11 and E13, five pairs of mammary buds develop along the mammary lines and in female embryos, by birth, each bud gives rise to a small mammary ductal tree with 15–20 branches. It is not until puberty that further development of the gland ensues. Under the influence of circulating growth hormone and estrogen, the distal end of each duct enlarges to form a terminal end bud (TEB), a specialized and highly proliferative epithelial structure. TEBs drive elongation and bifurcation of the ducts to form the mammary ductal tree, which spreads throughout the fatty stroma. With each estrous cycle, some subsequent alveolar differentiation occurs, but the formation of functionally differentiated mammary epithelial cells capable of producing milk only occurs during pregnancy and lactation. Early in pregnancy, the mammary epithelial ducts form side branches that serve as the ductules for grape-like alveolar clusters of differentiated mammary epithelial cells. Alveolar differentiation during pregnancy is driven by many factors, but circulating levels of progesterone and prolactin appear to play a dominant role. Full secretory differentiation and milk production only occur after parturition and appear to rely on a fall in progesterone levels in the setting of continued elevations in circulating levels of prolactin. Once lactation is completed the alveolar structures involute and the gland is remodeled back to a simple ductal tree in preparation for the next round of pregnancy. The capacity to undergo repeated cycles of alveolar differentiation and involution allows for repeated rounds of milk production to support multiple reproductive cycles.
Figure 3.
Morphological and schematic development of the mouse mammary gland during embryogenesis and postnatal life. (A) Cartoon illustrating the mammary line at 10.5 dpc in the mouse embryo, and the position of mammary rudiments in the 12.5 dpc mouse embryo. The mammary line forms transiently along the anterior-posterior axis at each flank of the embryo, eventually giving rise to 5 pairs of mammary placodes. There are three thoracic (1–3) and two inguinal (4 and 5) mammary buds. These buds give rise to a rudimental ductal tree by birth. (B) In the adult female mouse five pairs of mammary glands are found: three thoracic gland (1–3) and two inguinal glands (4 and 5). During puberty, hormones induce the rudimentry ductal network to elongate and proliferate. The ends of growing ducts form terminal end buds (TEB) during puberty (solid black ovals). The large oval shown in the postnatal stages depicts the mammary fat pad (stroma). During pregnancy, alveolar proliferation and differentiation occurs to produce milk proteins for mammary function during lactation. Mammary ducts are shown as solid lines and the lobuloalveolar structures are presented as solid black circles. Tissue remodeling and cell death occur during involution and bring the gland back to a virgin-like resting state. Scale bars in (A) embryo 1000 mm; (B) adult, 2500 mm.
Wnt Signaling During Mammary Gland Development
Embryonic development.
Wnt signaling is required for the formation of mammary glands in embryos. As noted above, in the mouse, two mammary lines form between the forelimb and the hindlimb at E10. By E12, cells within the mammary line have migrated and invaginated into the underlying mesenchyme to form five pairs of mammary buds in characteristic locations along the ventral aspect of the flank. These mammary buds contain pluripotent mammary stem cells competent to form a complete mammary ductal tree.
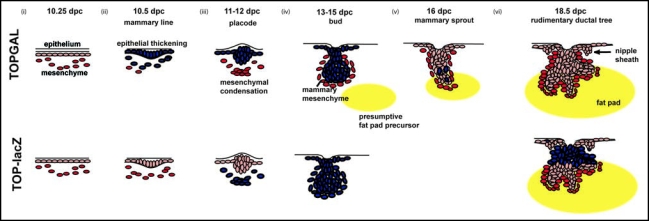
Several studies have made use of Wnt indicator mice which bear a β-catenin/TCF-responsive β-galactosidase transgene to determine which cells are targets of Wnt signaling during mammary development. TOPGAL mice and TOP-lacZ mice express identical transgenes, but have suggested slightly different patterns of Wnt activation (Fig. 4). Using TOPGAL mice, Chu et al., reported activation of Wnt signaling within cells in the embryonic mammary lines at the very inception of mammary development.48 TOPGAL expression remains active in the epithelial cells of mammary placodes and fully formed mammary buds. Once the mammary buds initiate ductal outgrowth, Wnt activity is considerably reduced within the epithelial compartment, although TOPGAL activity continues to be seen in scattered epithelial cells during the initial round of ductal morphogenesis in late embryonic life. Using TOP-lacZ mice, Boras-Granic et al., observed activation of canonical signaling first within the mesenchymal cells underlying the developing mammary placode on E11. Mesenchymal expression continued and epithelial expression was not seen until the buds were well formed at E13. Once the buds gave rise to ducts, epithelial expression was reduced, but remained in the remnants of the dense mammary mesenchyme just below the nipple. The reasons for these differences in expression patterns are not clear at this point but taken together these mice suggest that Wnt signaling is prominent in both epithelial cells and mesenchymal cells during the initiation of mammary development in embryos.
Figure 4.
Schematic overview of Wnt signaling during embryonic mammary gland development in the mouse. Initiation of mammary gland development proceeds through three inductive epithelial-mesenchymal signals. (i) Embryonic dermis instructs the overlying ectoderm to initiate placode formation. (ii) Epithelial placode signals to mesenchymal fibroblasts to form mesenchymal condensations. (iii) Mesenchymal condensations signal to the overlying bud to stimulate proliferation and downgrowth into the developing dermis. (iv) Subsequently, as the bud invaginates into the underlying mesenchyme, mammary mesenchymal cells surround the developing bud. A secondary mesenchyme, which gives rise to the future fat pad begins to develop. (v) The mammary sprout continues to proliferate and grow downward into the presumptive fat pad giving rise to a rudimentary ductal structure of 15–20 branches (vi). The nipple sheath also develops from the epidermal cells. Blue cells represent Wnt signalling activity during mammary gland development in TOPGAL (top) and TOP-lacZ transgenic embryos.
Functionally, it is clear that canonical Wnt signaling is necessary for the initiation of mammary development. Transgenic expression of the secreted Wnt inhibitor Dickkopf1, Dkk1, in the epidermis completely abolishes Wnt reporter activity in TOPGAL embryos and blocks the development of all of the mammary buds. Dkk1 expression prevents localized expression of all mammary placode markers48 and also inhibits molecular markers of the mammary line, such as Wnt10b. It is likely that Wnt6, Wnt3a or Wnt10b, all of which are broadly expressed within the flank epidermis on E10, may cooperate with Fgf10, Tbx3, Bmp4 and Neuregulin 3 to initiate the mammary line and upregulate specific Wnt10b expression, since these factors have also been implicated in the placement and initiation of mammary placode development.
In addition to the initiation of the mammary line, data from the TOPGAL and TOP-lacZ mice suggest that Wnt signaling may also be involved in the formation and/or maintenance of the placodes and mature buds as well. In support of this notion, the Wnt signaling mediator, Lef1, is required for development and maintenance of mammary buds. Although Lef1 is involved in the early specification of mammary placodes 2 and 3, the other placodes do form in Lef1-/- embryos, suggesting that Lef1 acts downstream of the formation of the mammary line. However, placodes 1, 4 and 5 subsequently degenerate in the absence of Lef1.49 Furthermore, TOP-lacZ/Lef1-/- crosses suggest that the lack of Lef1 inhibits activation of Wnt signaling in epithelial cells within the maturing mammary bud.49 Further support for a role of Wnt signaling in the mammary bud comes from recent experiments demonstrating smaller mammary placodes in embryos lacking Lrp5, the canonical Wnt ligand co-receptor.50 However, in Lrp5-/- mice, the buds do survive and give rise to ducts, although subsequent development is abnormal (see below).
Wnt signaling has been proposed to promote self-renewal and maintenance of stem cells. Therefore, Wnt signaling may be important for the specification or maintenance of progenitor cells in the mammary buds. The mammary developmental defects caused by the loss of Wnt signaling suggest that the stem cell population of the mammary gland may be compromised upon defective Wnt signaling. This is supported by the regression of buds in Lef1-deficient embryos and the failure of cells from Lrp5 deficient mice to generate new mammary glands in transplantation experiments.50 While the reasons for mammary bud deficiency in Lef1-/- mice remain unclear, it is possible that the defect is analogous to that seen in Tcf4-deficient mice, in which the stem cell compartments of intestinal crypts are severely depleted of progenitor cells with proliferative potential.51
While canonical Wnt signaling is activated in a subset of epithelial cells accompanying initial branch formation after E15–E16, whether Wnt signalling has a functional role at this stage has yet to be determined.48,50 Wnt signals are also localized around the nipple region and mesenchyme surrounding the primary duct at sites of localized Lef1 expression.49 Involvement of Wnt signaling during embryonic mammary sprout formation is further supported by studies in which overexpression of Wnt1 in the epithelium of embryonic mammary glands under the control of MMTV accelerates ductal development and branching while also bypassing androgen-mediated apoptosis in male mammary glands. To date, little is known about what regulates the initial phases of ductal growth, which, unlike ductal development during puberty, proceeds without input from systemic hormones. It is interesting to speculate if the mechanisms involved in the initial stages of branching morphogenesis during embryogenesis may help us in determining how breast cancers acquire hormone-independent growth during tumorigenesis.
Post-natal ductal development.
At the onset of puberty, ovarian and pituitary hormones induce rapid expansion and side-branching of the rudimentary ductal tree. During this phase, highly proliferating terminal end buds (TEBs), which are believed to house mammary stem cells, decorate the tips of growing ducts.52 Several Wnt genes show dynamic temporal and spatial expression patterns compatible with potential roles in regulating mammary gland development during puberty.53–55 Although several canonical signaling Wnts are expressed during puberty and in the adult mammary gland, expression of the Wnt reporters, TOPGAL and TOP-lacZ, is absent in mammary glands from birth through adolescence.48,49 Lindvall and colleagues noted expression of the BAT-GAL Wnt reporter transgene in a subset of epithelial cells in ducts during the first 2 weeks of life, but not thereafter. Nonetheless, they noted a severe impairment in ductal development during puberty in Lrp5-/- mice. Lack of Lrp5 led to a reduction in the numbers of TEBs, the rate of ductal penetration through the mammary fat pad and a reduction in the branching complexity of the gland. These data clearly suggest that the canonical pathway is important to proper ductal morphogenesis in response to hormonal stimulation during puberty. Given these data, however, the lack of activity of three different Wnt indicator transgenes during this period is curious. This may simply reflect a lack of sufficient sensitivity of these mice to detect low-level canonical Wnt activity in the mammary gland during puberty. Another possibility is that the defect in ductal development during puberty is a reflection of disruption of canonical Wnt signaling at an earlier stage, such as a reduction in stem cells as suggested by Lindvall et al. Therefore, the signficance of canonical Wnt signaling during puberty and in the adult virgin gland remains unclear.
While the above studies have focused on canonical Wnt signaling, data from Roarty and Serra have recently demonstrated that Wnt5a, a classical non-canonical Wnt, regulates normal ductal elongation and branching. It has been known for many years that TGFβ can act as an inhibitor of ductal development during puberty and it has been thought that TGFβ signaling was important to establishing proper ductal spacing. Roarty and Serra identified Wnt5a as a gene regulated by TGFβ in the mouse mammary gland. They showed that in the absence of Wnt5a, ductal development was accelerated and marked by the presence of larger TEBs, more extensive ductal invasion, increased lateral branching and elevated proliferation within the epithelium relative to wild-type glands.56 Furthermore, they demonstrated that Wnt5a is required for the inhibitory actions of TGFβ on ductal development. Thus, during normal development, Wnt5a may act downstream of TGFβ to facilitate proper growth and patterning of the ductal tree by acting as a negative regulator of extension and branching. Wnt5a has been shown to antagonize canonical Wnt signaling in several cell types, and it is possible that its role in normal development is to counteract canonical signaling during puberty and in the adult virgin.
Alveolar development.
Development of the mammary gland during pregnancy requires the formation of side branches off of the ductal tree, followed by the proliferation of alveolar units at the end of the newly formed terminal ductules. This process requires the coordination of cell proliferation, differentiation and morphogenesis and is regulated by circulating hormones. Progesterone appears to drive side branching and proliferation while the combination of progesterone and prolactin are thought to be necessary for alveolar cell differentiation.57 The previously described canonical Wnt signaling reporters, TOPGAL and TOP-lacZ are both active in mammary epithelial cells during pregnancy, suggesting that the canonical pathway is active during alveolar development.48,49 Furthermore, several genetic models of Wnt overexpression and inhibition suggest that Wnt signaling is functionally important during alveolar development. In early pregnancy, Wnt signaling may promote ductal side-branching,58 while later in pregnancy, canonical Wnt signals are essential for the proliferation and survival of lobuloalveolar progenitor cells.
Ectopic expression of several Wnts in mammary epithelial cells can prematurely induce a pregnancy phenotype in virgin mice. Expression of either Wnt1 or Wnt10b from an MMTV promoter induces ductal hyperbranching and precocious alveolar hyperplasia such that mammary glands of virgin transgenic mice resemble those of wild-type mice in mid-pregnancy, suggesting that constitutive Wnt expression bypasses the need for hormonal signals.59,60 Retroviral-mediated overexpression of Wnt4 also induces a ductal hyperbranching phenotype similar to that caused by Wnt1 and Wnt10b overexpression.61 Furthermore, progesterone induces expression of Wnt4 in the mammary epithelium during early to mid-pregnancy. Finally, transplantation of mammary buds from E14.5 Wnt4-/- embryos into the cleared fat-pads of syngeneic wild-type mice phenocopies the branching defects noted in progesterone receptor knockout ducts during pregnancy.58 In the aggregate, these data suggest that Wnt4 acts as a mediator of progesterone function during ductal side-branching in early pregnancy.53,55,62–64
Although overexpression of Wnts generally leads to ductal hyperplasia with alveolar differentiation, activation of the Wnt pathway by manipulating β-catenin levels and/or stability leads to alveolar differentiation without ductal side-branching or squamous transdifferentiation. Expression of an amino-terminally truncated and hence stabilized β-catenin using the MMTV promoter causes alveolar hyperplasia in virgin mice. Unlike the overexpression of Wnts, overexpression of stabilized β-catenin causes the direct budding of alveolar units from the sides of existing ducts, without the development of terminal ductules. These alveolar cells differentiate as evidenced by the production of lipid droplets and milk proteins, but do not proceed to a full secretory phenotype unless progesterone signaling is ablated.65 It appears that β-catenin and progesterone signaling interact in complicated ways and that only a subset of alveolar and/or ductal progenitor cells may be competent to respond to Wnt activation and that this responsiveness is, at least in some cells, progesterone dependant.65 Early alveolar expansion occurring as a result of transgenic β-catenin activation appears to occur independent of the actions of cyclin D1, which has been shown to be a classical canonical Wnt-responsive gene. However, full secretory differentiation of alveolar cells requires both canonical Wnt activation and cyclin D1. In contrast to the findings in these MMTV β-catenin models, when endogenous β-catenin is stabilized by recombination of a floxed exon that also encodes for a portion of the amino-terminus, mammary epithelial cells undergo a squamous pattern of skin and hair differentiation during pregnancy. The reason why this experiment and the simple overexpression of amino-terminally-truncated β-catenin give such divergent results remains unexplained. There may be subtle differences in the biochemistry of the β-catenin produced, the cell types activating β-catenin signaling or the absolute levels of β-catenin signaling achieved.63,64,66 In addition to the effects of activation of Wnt activation, inhibition of Wnt signaling has been shown to block alveolar differentiation during pregnancy and lead to lactational failure. Overexpression of the canonical Wnt inhibitor, Axin, in mammary epithelial cells inhibits alveolar cell expansion and differentiation during mid to late pregnancy, after the development of apparently normal side-branches. This was associated with a reduction in the expression of cyclin D1 and the induction of apoptosis in developing alveolar cells. Similar results were seen in response to the overexpression of a dominant negative β-catenin, β-engrailed (β-eng), which caused an impairment of alveolar cell expansion and differentiation after the formation of normal terminal ductules.67,68 In these mice, epithelial cell proliferation was reduced and apoptosis was increased, but cyclin D1 expression was not changed. These experiements demonstrate that endogenous β-catenin signaling is necessary for the normal expansion, survival and differentiation of alveolar progenitors during pregnancy, although it is not needed for the preceding ductal side-branching that occurs during early pregnancy.
In the aggregate, the data demonstrate that Wnt signaling is important to the development of the mammary gland during pregnancy. The differences in the various genetic models suggest that the ductal expansion that occurs in early pregnancy may result from the effects of Wnts acting on cells other than the luminal ductal epithelial cells, perhaps either the stromal cells or myoepithelial cells. Alternatively, these effects may be mediated by non-canonical Wnt signaling. It is clear that the canonical β-catenin pathway is necessary for the expansion, survival and differentiation of the milk-producing alveolar cells. However, it appears that not all luminal cells are equally responsive to activation of this pathway and that the phenotypes described above rely on specific interactions with other signaling pathways and the control of stem cell maintenance and renewal.
Conclusion
In summary, Wnt signaling is implicated at several stages of growth and differentiation of the mammary gland both during embryogenesis and after birth. To date, the canonical signaling pathway has been documented to have critical functions during mammary bud patterning and formation during embryogenesis as well as the development of the milk producing mammary gland during pregnancy. More recently, the noncanonical, β-catenin-independent pathway has been implicated during prepubertal and pubertal stages of development possibly through inhibition of the canonical Wnt signaling pathway. It will be interesting to see whether the dysregulation of noncanonical Wnt pathways may result in breast cancer as has been shown for the dysregulation of canoncical signaling. Clearly, this fascinating signaling system touches on many fundamental issues in mammary gland biology, such as morphogenesis, hormonal signaling, stem cell biology and most importantly, the development and pathogenesis of breast cancer. We have learned much in the last several years; we have much more to learn in the future.
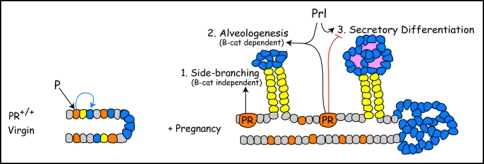
Figure 5.
Proposed interactions between progesterone receptor (PR) and β-catenin signaling during alveologenesis. In the normal virgin mammary gland, PR induces a non-uniform PR expression pattern and competence to respond to β-catenin in alveolar progenitors (blue) during ductal maturation. During early pregnancy, PR-Wnt4 signaling induces expansion of ductal progenitors (yellow) to form side-branches through βcatenin-independent routes. PR and PRL (prolactin) cooperate to induce alveologenesis, a process that is β-catenin-dependent. Later in pregnancy, PR restrains and PRL promote alveolar differentiation. β-catenin is required for alveologenesis and may participate at multiple steps in the secretory differentiation pathway.
Abbreviation
- APC
adenomatous polyposis coli
- β-cat
beta-catenin
- β-gal
beta-galactosidase
- β-TrCP
beta-transducin repeat-containing protein
- Bcl9
B-cell lymphoma 9
- BMP
bone morphogenic protein
- Ca2+
calcium CBP
- cAMP
response element-binding protein (CREB)-binding protein
- CKIα
casein kinase I alpha
- CtBP
carboxy-terminal binding protein
- Dkk
Dickkopf
- Dvl
Dishevelled
- Fgf
fibroblast growth factor
- Fzd
frizzled
- GAL
5-bromo-4-chloro-3-indolyl-D-galactoside
- GBP
glycogen synthase kinase 3 beta binding protein
- GMP
guanosine 5′monophosphate
- GSK3β
glycogen synthase kinase 3 beta
- GTPase
guanosine triphosphatase
- HBP1
HMG box repressor protein
- HDAC
histone deacetylase
- JNK
Jun-N-terminal kinase
- Krm
Kremen lacZ beta-galactosidase
- LDL
low density lipoprotein
- Lef1
Lymphoid enhancer factor 1
- LRP
low density lipoprotein receptor-related protein
- MAPK
mitogen activated protein kinase
- MMTV
mouse mammary tumour virus
- PIASy
protein inhibitor of STAT y
- PR
progesterone receptor
- PRL
prolactin Sfrp secreted frizzled related proteins
- Tbx
T-box (T = brachyury gene)
- Tcf
T cell factor TGFβ transforming growth factor beta
- Wnt
wingless-type
Footnotes
Previously published online as an Organogenesis E-publication: http://www.landesbioscience.com/journals/organogenesis/article/5858
References
- 1.Moon RT, Shah K. Developmental biology: Signaling polarity. Nature. 2002;417:239–240. doi: 10.1038/417239a. [DOI] [PubMed] [Google Scholar]
- 2.Bienz M, Clevers H. Armadillo/beta-catenin signals in the nucleus-proof beyond a reasonable doubt? Nat Cell Biol. 2003;5:179–182. doi: 10.1038/ncb0303-179. [DOI] [PubMed] [Google Scholar]
- 3.Alonso L, Fuchs E. Stem cells in the skin: waste not, Wnt not. Genes Dev. 2003;17:1189–1200. doi: 10.1101/gad.1086903. [DOI] [PubMed] [Google Scholar]
- 4.Staal FJ, Clevers HC. Wnt signaling in the thymus. Curr Opin Immunol. 2003;15:204–208. doi: 10.1016/s0952-7915(03)00003-7. [DOI] [PubMed] [Google Scholar]
- 5.Brennan KR, Brown AM. Wnt proteins in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2004;9:119–131. doi: 10.1023/B:JOMG.0000037157.94207.33. [DOI] [PubMed] [Google Scholar]
- 6.Katoh M. WNT/PCP signaling pathway and human cancer (review) Oncol Rep. 2005;14:1583–1588. [PubMed] [Google Scholar]
- 7.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westfall TA, Brimeyer R, Twedt J, Gladon J, Olberding A, Furutani-Seiki M, Slusarski DC. Wnt-5/pipetail functions in vertebrate axis formation as a negative regulator of Wnt/betacatenin activity. J Cell Biol. 2003;162:889–898. doi: 10.1083/jcb.200303107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 11.Strutt D. Frizzled signalling and cell polarisation in Drosophila and vertebrates. Development. 2003;130:4501–4513. doi: 10.1242/dev.00695. [DOI] [PubMed] [Google Scholar]
- 12.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 13.Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- 14.Yang-Snyder J, Miller JR, Brown JD, Lai CJ, Moon RT. A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr Biol. 1996;6:1302–1306. doi: 10.1016/s0960-9822(02)70716-1. [DOI] [PubMed] [Google Scholar]
- 15.Wehrli M, Dougan ST, Caldwell K, O'Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407:527–530. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- 16.Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 17.Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 18.Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, He X. A mechanism for Wnt coreceptor activation. Mol Cell. 2004;13:149–156. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- 19.Brannon M, Brown JD, Bates R, Kimelman D, Moon RT. XCtBP is a XTcf-3 corepressor with roles throughout Xenopus development. Development. 1999;126:3159–3170. doi: 10.1242/dev.126.14.3159. [DOI] [PubMed] [Google Scholar]
- 20.Levanon D, Goldstein RE, Bernstein Y, Tang H, Goldenberg D, Stifani S, Paroush Z, Groner Y. Transcriptional repression by AML1 and LEF-1 is mediated by the TLE/Groucho corepressors. Proc Natl Acad Sci USA. 1998;95:11590–11595. doi: 10.1073/pnas.95.20.11590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavallo RA, Cox RT, Moline MM, Roose J, Polevoy GA, Clevers H, Peifer M, Bejsovec A. Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature. 1998;395:604–608. doi: 10.1038/26982. [DOI] [PubMed] [Google Scholar]
- 22.Daniels DL, Weis WI. beta-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat Struct Mol Biol. 2005;12:364–371. doi: 10.1038/nsmb912. [DOI] [PubMed] [Google Scholar]
- 23.Chen G, Fernandez J, Mische S, Courey AJ. A functional interaction between the histone deacetylase Rpd3 and the corepressor groucho in Drosophila development. Genes Dev. 1999;13:2218–2230. doi: 10.1101/gad.13.17.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Billin AN, Thirlwell H, Ayer DE. Beta-catenin-histone deacetylase interactions regulate the transition of LEF1 from a transcriptional repressor to an activator. Mol Cell Biol. 2000;20:6882–6890. doi: 10.1128/mcb.20.18.6882-6890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hecht A, Kemler R. Curbing the nuclear activities of beta-catenin. Control over Wnt target gene expression. EMBO Rep. 2000;1:24–28. doi: 10.1093/embo-reports/kvd012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takemaru KI, Moon RT. The transcriptional coactivator CBP interacts with beta-catenin to activate gene expression. J Cell Biol. 2000;149:249–254. doi: 10.1083/jcb.149.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, Murone M, Zullig S, Basler K. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell. 2002;109:47–60. doi: 10.1016/s0092-8674(02)00679-7. [DOI] [PubMed] [Google Scholar]
- 28.Parker DS, Jemison J, Cadigan KM. Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila. Development. 2002;129:2565–2576. doi: 10.1242/dev.129.11.2565. [DOI] [PubMed] [Google Scholar]
- 29.Thompson B, Townsley F, Rosin-Arbesfeld R, Musisi H, Bienz M. A new nuclear component of the Wnt signalling pathway. Nat Cell Biol. 2002;4:367–373. doi: 10.1038/ncb786. [DOI] [PubMed] [Google Scholar]
- 30.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 31.Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun H, Taneja R. Stra13 expression is associated with growth arrest and represses transcription through histone deacetylase (HDAC)-dependent and HDAC-independent mechanisms. Proc Natl Acad Sci USA. 2000;97:4058–4063. doi: 10.1073/pnas.070526297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hovanes K, Li TW, Munguia JE, Truong T, Milovanovic T, Lawrence Marsh J, Holcombe RF, Waterman ML. Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet. 2001;28:53–57. doi: 10.1038/ng0501-53. [DOI] [PubMed] [Google Scholar]
- 34.Cadigan KM, Fish MP, Rulifson EJ, Nusse R. Wingless repression of Drosophila frizzled 2 expression shapes the Wingless morphogen gradient in the wing. Cell. 1998;93:767–777. doi: 10.1016/s0092-8674(00)81438-5. [DOI] [PubMed] [Google Scholar]
- 35.Baker JC, Beddington RS, Harland RM. Wnt signaling in Xenopus embryos inhibits bmp4 expression and activates neural development. Genes Dev. 1999;13:3149–3159. doi: 10.1101/gad.13.23.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prieve MG, Waterman ML. Nuclear localization and formation of beta-catenin-lymphoid enhancer factor 1 complexes are not sufficient for activation of gene expression. Mol Cell Biol. 1999;19:4503–4515. doi: 10.1128/mcb.19.6.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature. 2003;422:317–322. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 39.Rattner A, Hsieh JC, Smallwood PM, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci USA. 1997;94:2859–2863. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- 41.Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, Glinka A, Niehrs C. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 42.Uren A, Reichsman F, Anest V, Taylor WG, Muraiso K, Bottaro DP, Cumberledge S, Rubin JS. Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. J Biol Chem. 2000;275:4374–4382. doi: 10.1074/jbc.275.6.4374. [DOI] [PubMed] [Google Scholar]
- 43.Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang HY, Malbon CC. Wnt signaling, Ca2+ and cyclic GMP: visualizing Frizzled functions. Science. 2003;300:1529–1530. doi: 10.1126/science.1085259. [DOI] [PubMed] [Google Scholar]
- 45.Rulifson EJ, Wu CH, Nusse R. Pathway specificity by the bifunctional receptor frizzled is determined by affinity for wingless. Mol Cell. 2000;6:117–126. [PubMed] [Google Scholar]
- 46.Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- 47.Sakakura T. New aspects of stroma-parenchyma relations in mammary gland differentiation. Int Rev Cytol. 1991;125:165–202. doi: 10.1016/s0074-7696(08)61219-x. [DOI] [PubMed] [Google Scholar]
- 48.Chu EY, Hens J, Andl T, Kairo A, Yamaguchi TP, Brisken C, Glick A, Wysolmerski JJ, Millar SE. Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. Development. 2004;131:4819–4829. doi: 10.1242/dev.01347. [DOI] [PubMed] [Google Scholar]
- 49.Boras-Granic K, Chang H, Grosschedl R, Hamel PA. Lef1 is required for the transition of Wnt signaling from mesenchymal to epithelial cells in the mouse embryonic mammary gland. Dev Biol. 2006;295:219–231. doi: 10.1016/j.ydbio.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 50.Lindvall C, Evans NC, Zylstra CR, Li Y, Alexander CM, Williams BO. The Wnt signaling receptor Lrp5 is required for mammary ductal stem cell activity and Wnt1 induced tumorigenesis. J Biol Chem. 2006;281:35081–35087. doi: 10.1074/jbc.M607571200. [DOI] [PubMed] [Google Scholar]
- 51.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H, Willert K, Molenaar M, Roose J, Wagenaar G, Markman M, Lamers W, Destree O, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 52.Smalley M, Ashworth A. Stem cells and breast cancer: A field in transit. Nat Rev Cancer. 2003;3:832–844. doi: 10.1038/nrc1212. [DOI] [PubMed] [Google Scholar]
- 53.Gavin BJ, McMahon JA, McMahon AP. Expression of multiple novel Wnt-1/int-1related genes during fetal and adult mouse development. Genes Dev. 1990;4:2319–2332. doi: 10.1101/gad.4.12b.2319. [DOI] [PubMed] [Google Scholar]
- 54.Weber-Hall SJ, Phippard DJ, Niemeyer CC, Dale TC. Developmental and hormonal regulation of Wnt gene expression in the mouse mammary gland. Differentiation. 1994;57:205–214. doi: 10.1046/j.1432-0436.1994.5730205.x. [DOI] [PubMed] [Google Scholar]
- 55.Kouros-Mehr H, Werb Z. Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev Dyn. 2006;235:3404–3412. doi: 10.1002/dvdy.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roarty K, Serra R. Wnt5a is required for proper mammary gland development and TGF{beta}-mediated inhibition of ductal growth. Development. 2007;134:3929–3939. doi: 10.1242/dev.008250. [DOI] [PubMed] [Google Scholar]
- 57.Hennighausen L, Robinson GW. Think globally, act locally: the making of a mouse mammary gland. Genes Dev. 1998;12:449–455. doi: 10.1101/gad.12.4.449. [DOI] [PubMed] [Google Scholar]
- 58.Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK, McMahon JA, McMahon AP, Weinberg RA. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 2000;14:650–654. [PMC free article] [PubMed] [Google Scholar]
- 59.Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988;55:619–625. doi: 10.1016/0092-8674(88)90220-6. [DOI] [PubMed] [Google Scholar]
- 60.Lane TF, Leder P. Wnt-10b directs hypermorphic development and transformation in mammary glands of male and female mice. Oncogene. 1997;15:2133–2144. doi: 10.1038/sj.onc.1201593. [DOI] [PubMed] [Google Scholar]
- 61.Bradbury JM, Edwards PA, Niemeyer CC, Dale TC. Wnt-4 expression induces a pregnancy-like growth pattern in reconstituted mammary glands in virgin mice. Dev Biol. 1995;170:553–563. doi: 10.1006/dbio.1995.1236. [DOI] [PubMed] [Google Scholar]
- 62.Benhaj K, Akcali KC, Ozturk M. Redundant expression of canonical Wnt ligands in human breast cancer cell lines. Oncol Rep. 2006;15:701–707. [PubMed] [Google Scholar]
- 63.Imbert A, Eelkema R, Jordan S, Feiner H, Cowin P. Delta N89 beta-catenin induces precocious development, differentiation, and neoplasia in mammary gland. J Cell Biol. 2001;153:555–568. doi: 10.1083/jcb.153.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miyoshi K, Shillingford JM, Le Provost F, Gounari F, Bronson R, von Boehmer H, Taketo MM, Cardiff RD, Hennighausen L, Khazaie K. Activation of beta -catenin signaling in differentiated mammary secretory cells induces transdifferentiation into epidermis and squamous metaplasias. Proc Natl Acad Sci USA. 2002;99:219–224. doi: 10.1073/pnas.012414099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hiremath M, Lydon JP, Cowin P. The pattern of {beta}-catenin responsiveness within the mammary gland is regulated by progesterone receptor. Development. 2007;134:3703–3712. doi: 10.1242/dev.006585. [DOI] [PubMed] [Google Scholar]
- 66.Michaelson JS, Leder P. beta-catenin is a downstream effector of Wnt-mediated tumorigenesis in the mammary gland. Oncogene. 2001;20:5093–5099. doi: 10.1038/sj.onc.1204586. [DOI] [PubMed] [Google Scholar]
- 67.Hsu SC, Galceran J, Grosschedl R. Modulation of transcriptional regulation by LEF-1 in response to Wnt-1 signaling and association with beta-catenin. Mol Cell Biol. 1998;18:4807–4818. doi: 10.1128/mcb.18.8.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tepera SB, McCrea PD, Rosen JM. A beta-catenin survival signal is required for normal lobular development in the mammary gland. J Cell Sci. 2003;116:1137–1149. doi: 10.1242/jcs.00334. [DOI] [PubMed] [Google Scholar]