Abstract
For centuries, the formation of vein patterns in the leaf has intrigued biologists, mathematicians and philosophers. In leaf development, files of vein-forming procambial cells emerge from seemingly homogeneous subepidermal tissue through the selection of anatomically inconspicuous preprocambial cells. Although the molecular details underlying the orderly differentiation of veins in the leaf remain elusive, gradually restricted transport paths of the plant hormone auxin have long been implicated in defining sites of vein formation. Several recent advances now appear to converge on a more precise definition of the role of auxin flow at different stages of vascular development. The picture that emerges is that of vein formation as a self-organizing, reiterative, auxin transport-dependent process.
Key words: arabidopsis, leaf development, polar auxin transport, procambium, vascular patterning
The vascular system of plants is a branching array of cell files extending through all organs.1 In dicot leaves, these vascular strands, or ‘veins’, are arranged in a ramified pattern that largely reflects the shape of the leaf (Fig. 1A).2,3 ‘Lateral veins’ branch from a conspicuous central vein (‘midvein’) that is continuous with the stem vasculature. In many species, lateral veins extend along the leaf edge to form ‘marginal veins’, which connect to adjacent lateral veins to form prominent closed loops. Finally, a series of ‘higher-order veins’ branch from midvein and loops and can either terminate in the lamina (‘free-ending veins’) or join two veins (‘connected veins’).
Figure 1.
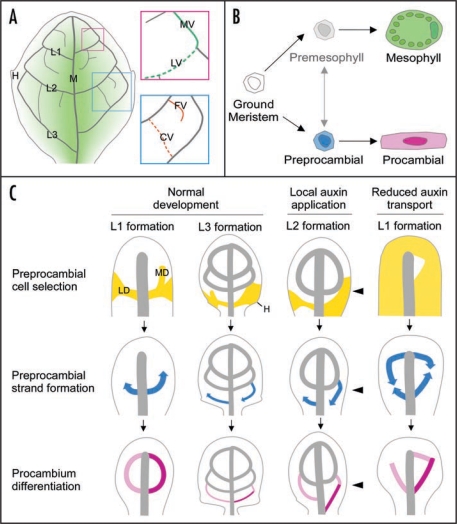
Conceptual summary of dicot leaf vein formation. (A) Schematics of a simplified mature leaf illustrating midvein (M), first, second and third loops (L1, L2 and L3, respectively)—each derived from corresponding lateral (LV) and marginal (MV) veins—free-ending (FV) and connected (CV) higher-order veins, hydathodes (H) and middle-to-margin positions (decreasing green gradient) as used in the text. (B) State transitions in leaf subepidermal cell differentiation. Available evidence suggests that the vein patterning process is limited to ground meristem cells (white), while subepidermal cells that have begun to acquire mesophyll characteristics are incapable of responding to vein-inducing signals.11,13,19,38 Expression of preprocambial (blue) and mesophyll emergence markers seem to identify two mutually exclusive and typically irreversible cell states, one leading to procambium (pink) and the other to mature mesophyll (green) formation. The transition from ground meristem to differentiated mesophyll could conceivably occur through a cell state that is formally equivalent to the preprocambial state in vascular differentiation. However, the existence of such a ‘premesophyll’ state (faded gray), the extent of its stability, its mutual exclusivity or competition with the preprocambial state and its responsiveness to vein-inducing signals still remain open questions. (C) Stage-specific dynamics of leaf vein patterning and their dependency on auxin levels and transport as exemplified for loop formation, but in general equally applicable to all veins. Upper series: PIN1-labeled auxin transport paths corresponding to preprocambial cell selection zones (yellow). Note how loops are composed of a lateral PIN1 expression domain (LD) and an initially free-ending marginal PIN1 expression domain (MD). Further, note slightly expanded PIN1 expression domains in a fraction of hydathode-associated third loops during normal development, broad PIN1 domains on the side of local auxin application (arrowhead) and nearly ubiquitous PIN1 expression upon systemic auxin transport inhibition. Middle series: directions of Athb8/J1721-marked preprocambial strand formation (blue arrows). Note middle-to-margin progression of preprocambial strand formation during normal loop development. Further, note margin-to-middle preprocambial strand extension in a fraction of third loops during normal development and in all loops forming on the side of auxin application. Finally, note co-existence of middle-to-margin and margin-to-middle polarities of preprocambial strand extension during the formation of individual loops in response to auxin transport inhibition. Lower series: gradual appearance of procambial cell identity acquisition (pink to magenta). Note simultaneous differentiation of lateral and marginal procambial strands in normal loop development. Further, note successive formation of lateral and marginal procambial strands in a fraction of third loops during normal development and in all loops formed on the side of auxin application and under conditions of reduced auxin transport. Arrows temporally connect successive stages of vein formation. See text for additional details.
Vascular cells mature from procambial cells: narrow, cytoplasmdense cells, characteristically arranged in continuous strands.4 Leaf procambial strands differentiate from files of isodiametric preprocambial cells, which are selected from the anatomically homogeneous subepidermal tissue of the leaf primordium, the ground meristem (Fig. 1B).5,6 The mechanism by which ground meristem cells are specified to procambial cell fate is unknown, but an instrumental role for auxin transport and resulting auxin distribution patterns in this process has increasingly gained support.7–13 This brief essay summarizes a recent group of articles that emphasizes the importance of auxin transport in leaf vein formation.
Vein Positioning and Preprocambial Cell Selection
Transport of the plant hormone auxin seems to be accurately visualized through the expression pattern and subcellular localization of auxin transport-associated proteins of the PIN family,14,15 and expression profiling identifies PIN1 as the most relevant member of the PIN gene family in leaf vein formation.11 During normal and experimentally altered leaf vein formation, PIN1 expression precedes and converges towards sites of preprocambial strand formation and, at the PIN1 expression level, all veins appear to be generated through two basic ontogenies (Fig. 1C).11,13 The midvein and lateral veins originate from subepidermal PIN1 domains associated with convergence points of PIN1 polarity in the epidermis, while higher-order veins emerge from PIN1 domains initiated within the expanding lamina. These internal domains are initially free-ending, but can become connected upon proximity to other PIN1 domains. Interestingly, each individual loop is composed of a ‘lateral’ PIN1 domain and a ‘marginal’ domain, which is ontogenically equivalent to a connected higher-order PIN1 domain (Fig. 1C). In mature leaves, the loops' composite origin is still recognizable in third and subsequent loop pairs, and it is only in the first and second loop pairs that this origin is obscured by the smooth amalgamation of lateral and marginal veins (Fig. 1A).11,16
Elevated auxin levels, whether naturally occurring in association with hydathodes or as a consequence of direct auxin application or auxin transport inhibition, lead to expanded PIN1 domains (Fig. 1C).10,11,13,17 Broad PIN1 domains eventually shrink to a few cell files predicting vein position, and the narrowing process is dependent on auxin transport (Fig. 1C). Within narrow PIN1 domains, subcellular localization indicates auxin transport towards pre-existing veins: in free-ending domains, a single polarity exists, while in connected domains, two opposite polarities are connected by a bipolar cell.11,13 PIN1 polarity may not be uniformly directed towards pre-existing veins across wide PIN1 domains, but it is usually so along each domain's midline.
These observations are consistent with the notion that vasculature is formed along core areas of gradually restricted domains of elevated auxin transport.7,18 Progressive restriction of expression domains to sites of vein formation has been shown for a number of genes implicated in vascular patterning,13,19–23 suggesting that this may represent a general feature of the preprocambial cell selection process.
Preprocambial Strand Formation
During the formation of all veins and under all tested experimental conditions, expression of preprocambial markers such as Athb8 and J1721 is initiated next to pre-existing vasculature and then extends progressively away from this point of origin,16,19,24 suggesting that all veins arise as free-ending preprocambial branches (Fig. 1C). Furthermore, connected veins are formed by fusion of initially free-ending preprocambial strands and free-ending veins result from termination of the extension of preprocambial expression domains (Fig. 1C).19,24
Although Athb8/J1721-expressing preprocambial strands extend progressively under all conditions, the specific direction of this progression varies. In fact, while preprocambial strands in the first and second loop pairs invariably extend from central to marginal regions of the developing leaf, third loop preprocambial strands can form in the opposite direction (i.e., marginal to central) (Fig. 1C). Unlike the first and second loop pairs, third loop pairs are associated with conspicuous auxin response maxima at the primordium margin and expanded PIN1 subepidermal domains (Fig. 1C).10,11,13,17 This suggests that Athb8/J1721 preprocambial strands are initiated at a critical auxin level, which for third and subsequent loop pairs could be reached at the margin of the primordium, possibly because of localized auxin synthesis.25 In contrast, auxin levels critical for preprocambial strand initiation for the first two loop pairs would be attained at the centre of the primordium, in proximity of the midvein, presumably the point of convergence of auxin produced anywhere in the lamina. This interpretation is further supported by the observation that direct auxin application at the primordium margin results in reversal of polarity of J1721 preprocambial strand formation, which then occurs exclusively from the margin to the middle of the primordium (Fig. 1C).24 Moreover, when auxin flow is systemically impaired throughout primordium development, individual loops can even be formed through the fusion of J1721 preprocambial strands that extend from central regions of the primordium towards its margins with J1721 strands that progress from the primordium margin towards its middle (Fig. 1C).24
Procambium Differentiation
Expression of procambial differentiation markers, including ET1335 and Q0990, suggests that procambium distinctive features appear simultaneously along entire strands (Fig. 1C).9,19,24 Preprocambial cells acquire the procambium-distinctive narrow shape through coordinated cell elongation occurring along the entire length of the strand, rather than through a synchronized cell division parallel to the axis of the preprocambial strand.24,26,27
Whereas procambium differentiates simultaneously throughout the first two loop pairs, lateral and marginal procambial strands can appear successively in third loops (Fig. 1C).24 As formation of third loop pairs is associated with increased auxin levels at the hydathode,10,11,17,25 excess auxin seems to prevent simultaneous procambium differentiation along entire loops. This hypothesis is also supported by the observation that separate appearance of lateral and marginal strands occurs in all loop-like veins formed in response to auxin application (Fig. 1C).24 Furthermore, when auxin levels are raised in the primordium because of reduced auxin drainage, lateral and marginal strands differentiate separately in all loops, including the typically simultaneously differentiating first two loop pairs (Fig. 1C).24 Therefore, given enough auxin at critical stages of development, formation of all procambial loops occurs in temporally distinct steps. Increased auxin levels could lead to deviations in simultaneity of procambial loop differentiation by delaying the transition of incipient veins (e.g., the lateral vein of the third loop) from less efficient to more efficient sinks. This, in turn, would prevent the formation of a connection (e.g., the marginal vein of the third loop) with pre-existing veins (e.g., the second loop).28 The simultaneous differentiation of lateral and marginal procambial strands in first and second loop pairs observed under normal conditions could thus simply reflect efficient auxin flow and/or inconspicuous auxin synthesis in early primordium development.
Termination of Vein Formation
Termination of vein formation could, in principle, occur at any developmental stage. However, available evidence suggests that, although formally possible, termination is unlikely to occur at the procambial stage. In fact, all mutants isolated to date that show fragmented procambial strands also display similar continuity defects at Athb8-labeled preprocambial stages.11,20,29–31 Furthermore, mutants with fewer procambial strands, a higher proportion of which end freely, show similar reduced complexity and connectivity at Athb8-expressing preprocambial stages.32–35 These observations suggest that termination of procambial strands must have occurred at preprocambial stages. Termination of preprocambial strand formation has, in turn, been suggested to occur because of exhausted signaling within the developing veins20,31,36,37 and/or because of halted proliferation and consequent differentiation of the remaining ground meristem population into the alternative subepidermal tissue of the mature leaf, the mesophyll.19,38 Whether these different mechanisms are parts of the same pathway will have to await the identification of more molecular players.
Conclusions and Perspectives
How vein patterns can be generated has been subject to discussion, and although pieces of the puzzle have started to emerge, many questions remain unanswered. Different stages of vein formation display strikingly different dynamics (Fig. 1C). How are dynamics at a specific stage translated into those characteristic of the successive stage? Expression of preprocambial and mesophyll emergence markers seems to identify two non-overlapping cell states (Fig. 1B). How are developmental decisions made in vein-forming cells coordinated with decisions made in non-vascular tissues? While the answers to these and other questions still elude us, it is clear that an in-depth understanding of vein formation is a prerequisite to answering them. The recent work described here is a step in that direction.
Acknowledgements
We thank Thomas Berleth, Nancy Dengler, Julie Kang and Danielle Marcos for invaluable comments on the manuscript. This work was supported by a Discovery Grant of the Natural Sciences and Engineering Research Council of Canada (NSERC), by an Alberta Ingenuity (AI) New Faculty Grant and by the Canada Research Chairs Program. TJD was supported by an NSERC USRA, an NSERC CGS-M Scholarship, and an AI Student Scholarship.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5345
References
- 1.Esau K. Plant anatomy. New York ; London: John Wiley; 1965. [Google Scholar]
- 2.Dengler N, Kang J. Vascular patterning and leaf shape. Curr Opin Plant Biol. 2001;4:50–56. doi: 10.1016/s1369-5266(00)00135-7. [DOI] [PubMed] [Google Scholar]
- 3.Nelson T, Dengler N. Leaf vascular pattern formation. Plant Cell. 1997;9:1121–1135. doi: 10.1105/tpc.9.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esau K. Origin and development of primary vascular tissues in plants. Bot Rev. 1943;9:125–206. [Google Scholar]
- 5.Foster AS. Foliar venation in angiosperms from an ontogenetic standpoint. Am J Bot. 1952;39:752–766. [Google Scholar]
- 6.Pray TR. Foliar venation of Angiosperms. IV. Histogenesis of the venation of Hosta. Am J Bot. 1955;42:698–706. [Google Scholar]
- 7.Sachs T. The control of the patterned differentiation of vascular tissues. Adv Bot Res. 1981;9:151–262. [Google Scholar]
- 8.Sachs T. The development of vascular networks during leaf development. Curr Top Plant Biochem Physiol. 1989;8:168–183. [Google Scholar]
- 9.Mattsson J, Sung ZR, Berleth T. Responses of plant vascular systems to auxin transport inhibition. Development. 1999;126:2979–2991. doi: 10.1242/dev.126.13.2979. [DOI] [PubMed] [Google Scholar]
- 10.Mattsson J, Ckurshumova W, Berleth T. Auxin signaling in Arabidopsis leaf vascular development. Plant Physiol. 2003;131:1327–1339. doi: 10.1104/pp.013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scarpella E, Marcos D, Friml J, Berleth T. Control of leaf vascular patterning by polar auxin transport. Genes Dev. 2006;20:1015–1027. doi: 10.1101/gad.1402406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sieburth LE. Auxin is required for leaf vein pattern in Arabidopsis. Plant Physiol. 1999;121:1179–1190. doi: 10.1104/pp.121.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wenzel CL, Schuetz M, Yu Q, Mattsson J. Dynamics of MONOPTEROS and PIN-FORMED1 expression during leaf vein pattern formation in Arabidopsis thaliana. Plant J. 2007;49:387–398. doi: 10.1111/j.1365-313X.2006.02977.x. [DOI] [PubMed] [Google Scholar]
- 14.Wisniewska J, Xu J, Seifertova D, Brewer PB, Ruzicka K, Blilou I, Rouquie D, Benkova E, Scheres B, Friml J. Polar PIN localization directs auxin flow in plants. Science. 2006;312:883. doi: 10.1126/science.1121356. [DOI] [PubMed] [Google Scholar]
- 15.Petrasek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertova D, Wisniewska J, Tadele Z, Kubes M, Covanova M, Dhonukshe P, Skupa P, Benkova E, Perry L, Krecek P, Lee OR, Fink GR, Geisler M, Murphy AS, Luschnig C, Zazimalova E, Friml J. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science. 2006;312:914–918. doi: 10.1126/science.1123542. [DOI] [PubMed] [Google Scholar]
- 16.Kang J, Dengler N. Vein pattern development in adult leaves of Arabidopsis thaliana. Int J Plant Sci. 2004;165:231–242. [Google Scholar]
- 17.Aloni R, Schwalm K, Langhans M, Ullrich CI. Gradual shifts in sites of free-auxin production during leaf-primordium development and their role in vascular differentiation and leaf morphogenesis in Arabidopsis. Planta. 2003;216:841–853. doi: 10.1007/s00425-002-0937-8. [DOI] [PubMed] [Google Scholar]
- 18.Rolland Lagan AG, Prusinkiewicz P. Reviewing models of auxin canalization in the context of leaf vein pattern formation in Arabidopsis. Plant J. 2005;44:854–865. doi: 10.1111/j.1365-313X.2005.02581.x. [DOI] [PubMed] [Google Scholar]
- 19.Scarpella E, Francis P, Berleth T. Stage-specific markers define early steps of procambium development in Arabidopsis leaves and correlate termination of vein formation with mesophyll differentiation. Development. 2004;131:3445–3455. doi: 10.1242/dev.01182. [DOI] [PubMed] [Google Scholar]
- 20.Carland FM, Nelson T. Cotyledon vascular pattern2-mediated inositol (1,4,5) triphosphate signal transduction is essential for closed venation patterns of Arabidopsis foliar organs. Plant Cell. 2004;16:1263–1275. doi: 10.1105/tpc.021030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardtke CS, Berleth T. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 1998;17:1405–1411. doi: 10.1093/emboj/17.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawa S, Koizumi K, Naramoto S, Demura T, Ueda T, Nakano A, Fukuda H. DRP1A is responsible for vascular continuity synergistically working with VAN3 in Arabidopsis. Plant Physiol. 2005;138:819–826. doi: 10.1104/pp.105.061689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geldner N, Richter S, Vieten A, Marquardt S, Torres Ruiz RA, Mayer U, Jurgens G. Partial loss-of-function alleles reveal a role for GNOM in auxin transport-related, post-embryonic development of Arabidopsis. Development. 2004;131:389–400. doi: 10.1242/dev.00926. [DOI] [PubMed] [Google Scholar]
- 24.Sawchuk MG, Head P, Donner TJ, Scarpella E. Time-lapse imaging of Arabidopsis leaf development shows dynamic patterns of procambium formation. New Phytol. 2007;176:560–571. doi: 10.1111/j.1469-8137.2007.02193.x. [DOI] [PubMed] [Google Scholar]
- 25.Cheng Y, Dai X, Zhao Y. Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev. 2006;20:1790–1799. doi: 10.1101/gad.1415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donnelly PM, Bonetta D, Tsukaya H, Dengler RE, Dengler NG. Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev Biol. 1999;215:407–419. doi: 10.1006/dbio.1999.9443. [DOI] [PubMed] [Google Scholar]
- 27.Kang J, Dengler N. Cell cycling frequency and expression of the homeobox gene ATHB-8 during leaf vein development in Arabidopsis. Planta. 2002;216:212–219. doi: 10.1007/s00425-002-0847-9. [DOI] [PubMed] [Google Scholar]
- 28.Sachs T. On determination of pattern of vascular tissues in peas. Ann Bot. 1968;32:781–790. [Google Scholar]
- 29.Deyholos MK, Cordner G, Beebe D, Sieburth LE. The SCARFACE gene is required for cotyledon and leaf vein patterning. Development. 2000;127:3205–3213. doi: 10.1242/dev.127.15.3205. [DOI] [PubMed] [Google Scholar]
- 30.Koizumi K, Sugiyama M, Fukuda H. A series of novel mutants of Arabidopsis thaliana that are defective in the formation of continuous vascular network: calling the auxin signal flow canalization hypothesis into question. Development. 2000;127:3197–3204. doi: 10.1242/dev.127.15.3197. [DOI] [PubMed] [Google Scholar]
- 31.Carland FM, Berg BL, FitzGerald JN, Jinamornphongs S, Nelson T, Keith B. Genetic regulation of vascular tissue patterning in Arabidopsis. Plant Cell. 1999;11:2123–2137. doi: 10.1105/tpc.11.11.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petricka JJ, Nelson TM. Arabidopsis nucleolin affects plant development and patterning. Plant Physiol. 2007;144:173–186. doi: 10.1104/pp.106.093575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alonso-Peral MM, Candela H, del Pozo JC, Martinez-Laborda A, Ponce MR, Micol JL. The HVE/CAND1 gene is required for the early patterning of leaf venation in Arabidopsis. Development. 2006;133:3755–3766. doi: 10.1242/dev.02554. [DOI] [PubMed] [Google Scholar]
- 34.Candela H, Martinez-Laborda A, Micol JL. Venation pattern formation in Arabidopsis thaliana vegetative leaves. Dev Biol. 1999;205:205–216. doi: 10.1006/dbio.1998.9111. [DOI] [PubMed] [Google Scholar]
- 35.Cnops G, Neyt P, Raes J, Petrarulo M, Nelissen H, Malenica N, Luschnig C, Tietz O, Ditengou F, Palme K, Azmi A, Prinsen E, Van Lijsebettens M. The TORNADO1 and TORNADO2 genes function in several patterning processes during early leaf development in Arabidopsis thaliana. Plant Cell. 2006;18:852–866. doi: 10.1105/tpc.105.040568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steynen QJ, Schultz EA. The FORKED genes are essential for distal vein meeting in Arabidopsis. Development. 2003;130:4695–4708. doi: 10.1242/dev.00689. [DOI] [PubMed] [Google Scholar]
- 37.Motose H, Sugiyama M, Fukuda H. A proteoglycan mediates inductive interaction during plant vascular development. Nature. 2004;429:873–878. doi: 10.1038/nature02613. [DOI] [PubMed] [Google Scholar]
- 38.Kang J, Mizukami Y, Wang H, Fowke L, Dengler NG. Modification of cell proliferation patterns alters leaf vein architecture in Arabidopsis thaliana. Planta. 2007;226:1207–1218. doi: 10.1007/s00425-007-0567-2. [DOI] [PubMed] [Google Scholar]