Abstract
GCR2 was recently proposed to represent a G-protein-coupled receptor (GPCR) for the plant hormone, abscisic acid (ABA). We and others provided evidence that GCR2 is unlikely to be a bona fide GPCR because it is not clearly predicted to contain seven transmembrane domains, a structural hallmark for classical GPCRs. Instead, GCR2 shows significant sequence similarity to homologs of bacterial lanthionine synthetase component C (LanC). Here, we provide additional analysis of GCR2 and LanC-like (LANCL) proteins in plants, and propose that GCR2 is a new member of the eukaryotic LANCL protein family.
Key words: GCR2, G-protein-coupled receptor, abscisic acid (ABA), lanthionine synthetase
Seven transmembrane (7TM) G-protein-coupled receptors (GPCRs) comprise the largest protein family in mammals, and are the most pharmacologically important receptor family, being the target of approximately half of all modern medicinal drugs. All canonical GPCRs are integral membrane proteins and are predicted to contain 7TM-spanning domains as their structural hallmark, a pattern confirmed by the high-resolution crystal structure of human β2-adrenergic GPCR.1,2 GPCRs sense extracellular molecules and activate intracellular cell signaling via coupling with heterotrimeric G-proteins. Heterotrimeric G-protein subunits are conserved in plants, but the repertoire of heterotrimeric G-protein complexes to which they contribute in plants is much simpler than in mammals.3,4 Liu et al. (2007) proposed that GCR2 is a GPCR for the plant hormone abscisic acid (ABA) in Arabidopsis.5 However, GCR2 was predicted not to be a 7TM protein when its amino acid sequence was analyzed in robust transmembrane prediction systems.6,7 On the other hand, GCR2 has significant sequence similarity to homologs of bacterial lanthionine synthetase component C (LanC) that are found in diverse eukaryotes and which have predicted structural similarity to prokaryotic LanC.6,7 These findings raise the possibility that GCR2 belongs to the LanC protein superfamily, rather than the GPCR superfamily.
Definition of LanC-Like Proteins
Prokaryotic LanC protein is a part of a multimeric membrane-associated lanthionine synthetase complex involved in the modification and transport of peptides. LanC itself is a zinc-containing enzyme that acts in concert with specific dehydratases to facilitate intramolecular conjugation of cysteine to serine or threonine residues, yielding macrocyclic thioether (lanthionine) products. These display potent antimicrobial activity, and are known as lantibiotics.8–10
The first member of the eukaryotic LanC-like (LANCL) protein family, LANCL1, was isolated from human erythrocyte membranes.11 A related protein, LANCL2, was subsequently identified in humans.12 LANCL1 homologs have also been found and characterized in other mammals, including mouse, rat and cattle.13–16 Comparison of the amino acid sequence of eukaryotic LANCL proteins with prokaryotic LanC enzymes revealed several critical similarities, and these have formed the foundation for the definition of LANCL proteins in eukaryotes.15,17 First, LANCL proteins contain seven hydrophobic sequence repeats, each with one characteristic GXXG consensus motif (where G = glycine and X = any amino acid). Second, LANCL proteins contain three critical motifs, a histidine (H)-glycine (G) motif in repeat 4, a tryptophan (W)-cysteine (C)-X-glycine (G) motif in repeat 5, and a cysteine (C)-histidine (H)-glycine (G) motif in repeat 6. As revealed by the crystal structure of the Lactococcus lactis LanC protein, nisin cyclise (NisC), and by site-directed mutagenesis studies,18,19 the HG motif of repeat 4 is critical for substrate deprotonation in order to allow correct cyclization, and the WCXG motif of repeat 5 and CHG motif of repeat 6 contain conserved zinc-coordinating resides. It is important to bear in mind that eukaryotic LANCL proteins typically only show low identities (∼ 20%) with prokaryotic LanC enzymes at the amino acid level, but the GXXG motifs are largely conserved and residues in the HG, WCXG and CHG motifs are invariant.
GCR2 is a Member of LanCL Protein Family
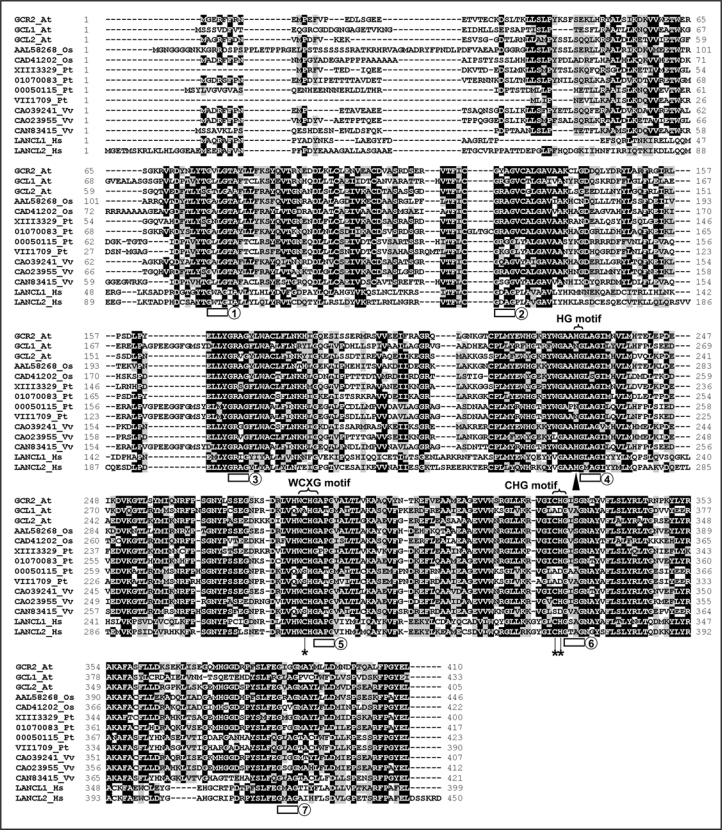
Arabidopsis GCR2encodes a protein with 410 amino acids. The seven GXXG motifs, HG, WCXG and CHG motifs are completely conserved in GCR2, satisfying the key criteria for GCR2 being a member of LANCL protein family (Fig. 1). There are two additional GCR2-like proteins, GCR2-LIke 1 (GCL1) and GCR2-LIke 2 (GCL2), encoded in the Arabidopsis genome.6 Sequence homologs of GCR2 are also present in the sequenced genomes of rice (Oryza sativa), poplar (Populus trichocarpa) and grape (Vitis vinifera), including at least two GCR2 homologs encoded in each of these genomes. All of these proteins are highly similar to the human LANCL1 and LANCL2 proteins (Fig. 1). The seven GXXG motifs are entirely conserved in all of these proteins. However, the HG motif in repeat 4, WCXG motif in repeat 5, and CHG motif in repeat 6 contain variants in one of the two GCR2 homologs (GCL1) in Arabidopsis, in two of the four GCR2 homologs in poplar, and in one of the three GCR2 homologs detected in grape (Fig. 1). Based on the crystal structure and site-directed mutagenesis study of NisC,18,19 the H residue of the HG motif in repeat 4 is thought to be critical for correct substrate cyclization, and the C residue of the WCXG motif in repeat 5 and C and H residues of the CHG motif in repeat 6 are essential for NisC's enzymatic activity. It is therefore possible that proteins containing substituted residues in these motifs may have evolved to differ functionally from proteins that retain the normally invariant residues at those locations.
Figure 1.
Amino acid sequence of GCR2 and multiple alignments with other lanthionine synthetase component C (LanC)-like proteins in plants and in humans. The amino acid sequences were aligned by CLUSTALW. Amino acids that are identical or similar are shaded with black or gray, respectively. The seven GXXG motifs are indicated by blocks underneath, and are numbered 1 to 7. The H reside of the HG motif in repeat 4 that is critical for substrate de-protonation for correct cyclization as shown in the crystal structure of the Lactococcus lactis LanC protein, nisin cyclise (NisC), and by site-directed mutagenesis studies,18,19 is indicated by a triangle underneath. The C residue of the WCXG motif of repeat 5 and the C and H residues in the CHG motif of repeat 6 that comprise conserved zinc-coordinating resides and are essential for the enzymatic activity of NisC, are indicated by stars underneath. The proteins aligned are (name of species and accession number in parentheses): GCR2_At (Arabidopsis thaliana, NP_175700), GCL1_At (Arabidopsis thaliana, NP_201331), GCL2_At (Arabidopsis thaliana, NP_850003), AAL58268_Os (Oryza sativa, AAL58268), CAD41202_Os (Oryza sativa, CAD41202), XIII3329_Pt (Populus trichocarpa, estExt_Genewise1_v1.C_LG_XIII3329), 01070083_Pt (Populus trichocarpa, eugene3.01070083), 00050115_Pt (Populus trichocarpa, eugene3.00050115), VIII1709_Pt (Populus trichocarpa, estExt_Genewise1_v1.C_LG_VII1709), CAO39241_Vv (Vitis vinifera, CAO39241), CAO23955_Vv (Vitis vinifera, CAO23955), CAN83415_Vv (Vitis vinifera, CAN83415), LANCL1_Hs (Homo sapiens, NP_006046) and LANCL2_Hs (Homo sapiens, NP_061167).
Functional Characterization of LANCL Proteins in Mammals
The presence of macrocyclic lanthionine-containing polypeptides similar to the prokaryotic lantibiotic structures has not been documented in higher eukaryotes, thus leaving the exact biological role of LANCL proteins unclear. The functional characterization of LANCL proteins is still in its infancy, and has been mainly conducted in mammalian species. These analyses have been limited to gene expression profiling, subcellular localization, overexpression phenotyping, and biochemical characterization. The human genome encodes three LANCL proteins, LANCL1, LANCL2 and LANCL3,20 but only LANCL1 and LANCL2 have been characterized to any degree. The LANCL1 and LANCL2 genes have similar expression patterns, with strong expression in brain and testis, and weak ubiquitous expression in other tissues.11,12 Because testis and brain are organs separated by blood-tissue barriers, it has been speculated that LANCL1 may play a role in the immune surveillance of these organs in rat.15 Consistent with this view, in the bovine central nervous system, LANCL1 binds glutathione, a very abundant cellular sulfhydryl-containing molecule that is responsible for maintenance of a reducing intracellular environment and for protection against electrophilic toxins,16 leading to the speculation that LANCL1 may have a role in neurodegenerative disease.16 On the other hand, LANCL1 was found to be central for the development of malarial parasite Plasmodium falciparum, where it participates in the assembly of the cytoadherence complex, essential for the pathogenesis of cerebral malaria.21
LANCL2 is most highly expressed in testis in mammals, and introduction of exogenous LANCL2 has been shown to cause increased cellular sensitivity to the anticancer drug, andriamycin, by suppressing the expression of MULTIDRUG-RESISTANCE 1 (MDR1) and its cognate protein, P-glycoprotein. These attributes have led to LANC2's designation as ‘Testis Adriamycin Sensitivity Protein’.22 Interestingly, overexpressed LANCL2 was found to interact with the cortical actin cytoskeleton, implying that LANCL2 may also have a role in cytoskeleton reorganization.20
It is worthwhile to note that LANCL1 was also initially proposed as a GPCR due to its seven predicted hydrophobic α-helices.11 However, subsequent biochemical analysis revealed that the LANCL1 is not an integral membrane protein, but rather a loosely bound peripheral membrane protein.17 Consistent with this, LANCL1 proteins are mainly found in the cytosol and nucleus.16,17,20 The LANCL2 protein is also found in the cytosol and nucleus, but can be associated with the plasma membrane, as well.20,22 However, the membrane localization of LANCL2 is due to myristoylation-based lipid binding rather than possession of classical TM domains.20
Speculation: Function of GCR2 in Arabidopsis
As in mammals, lantibiotics have not been found in plants. It is therefore possible that plant LANCL proteins may have evolved some functions that are distinct from the original function of prokaryotic LanC enzymes. Putative loss-of-function alleles of GCR2 did not display any obvious morphological defects,6 offering little clue to the exact biological role of GCR2 in Arabidopsis. One possible explanation for the lack of a deficiency phenotype is that functional redundancy exists among GCR2, GCL1 and GCL2 genes. examination of mutants carrying a combination of loss-of-function mutations in these genes may help clarify this possibility. The other possible explanation is that gcr2 mutants may have subtle phenotypes, or may only exhibit phenotypes under certain conditions. For example, gcr2 mutants were shown to have insensitivity to ABA in seed germination and early seedling development under one experimental condition5 but displayed wild-type response to ABA under another experimental condition.6 elucidation of the exact role of GCR2 in ABA signaling or metabolism therefore awaits further investigation. Liu et al. (2007) have speculated that GCR2, a LANCL protein, may define a new type, nonclassical GPCR.23 This idea, which might be consistent with the concept that plant LANCL homologs have evolved to fulfill novel functions, certainly needs to be tested further.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/5292
References
- 1.Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-resolution crystal structure of an engineered human β2-adrenergic G protein coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Yao XJ, Weis WI, Stevens RC, Kobilka BK. GPCR engineering yields high-resolution structural insights into β2 adrenergic receptor function. Science. 2007;318:1266–1273. doi: 10.1126/science.1150609. [DOI] [PubMed] [Google Scholar]
- 3.Assmann SM. Heterotrimeric and unconventional GTP binding proteins in plant cell signaling. Plant Cell. 2002;14:S355–S373. doi: 10.1105/tpc.001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Temple BRS, Jones AM. The plant heterotrimeric G-protein complex. Annu Rev Plant Biol. 2007;58:249–266. doi: 10.1146/annurev.arplant.58.032806.103827. [DOI] [PubMed] [Google Scholar]
- 5.Liu X, Yue Y, Li B, Nie Y, Li W, Wu WH, Ma L. A G protein-coupled receptor is a plasma membrane receptor for the plant hormone abscisic acid. Science. 2007;315:1712–1716. doi: 10.1126/science.1135882. [DOI] [PubMed] [Google Scholar]
- 6.Gao Y, Zeng Q, Guo J, Cheng J, Ellis BE, Chen JG. Genetic characterization reveals no role for the reported ABA receptor, GCR2, in ABA control of seed germination and early seedling development in Arabidopsis. Plant J. 2007;52:1001–1013. doi: 10.1111/j.1365-313X.2007.03291.x. [DOI] [PubMed] [Google Scholar]
- 7.Johnston CA, Temple BR, Chen JG, Gao Y, Moriyama EN, Jones AM, Siderovski DP, Willard FS. Comment on “A G protein-coupled receptor is a plasma membrane receptor for the plant hormone abscisic acid”. Science. 2007;318:914c. doi: 10.1126/science.1143230. [DOI] [PubMed] [Google Scholar]
- 8.Siegers K, Heinzmann S, Entian KD. Biosynthesis of lantibiotic nisin. Posttranslational modification of its prepeptide occurs at a multimeric membrane-associated lanthionine synthetase complex. J Biol Chem. 1996;271:12294–12301. doi: 10.1074/jbc.271.21.12294. [DOI] [PubMed] [Google Scholar]
- 9.Sahl HG, Bierbaum G. Lantibiotics: Biosynthesis and biological activities of uniquely modified peptides from gram-positive bacteria. Annu Rev Microbiol. 1998;52:41–79. doi: 10.1146/annurev.micro.52.1.41. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee C, Paul M, Xie L, van der Donk WA. Biosynthesis and mode of action of lantibiotics. Chem Rev. 2005;105:633–684. doi: 10.1021/cr030105v. [DOI] [PubMed] [Google Scholar]
- 11.Mayer H, Salzer U, Breuss J, Ziegler S, Marchler-Bauer A, Prohaska R. Isolation, molecular characterization, and tissue-specific expression of a novel putative G protein-coupled receptor. Biochim Biophys Acta. 1998;1395:301–308. doi: 10.1016/s0167-4781(97)00178-4. [DOI] [PubMed] [Google Scholar]
- 12.Mayer H, Pongratz M, Prohaska R. Molecular cloning, characterization, and tissue-specific expression of human LANCL2, a novel member of the LanC-like protein family. DNA Seq. 2001;12:161–166. doi: 10.3109/10425170109080770. [DOI] [PubMed] [Google Scholar]
- 13.Mayer H, Breuss J, Ziegler S, Prohaska R. Molecular characterization and tissue-specific expression of a murine putative G-protein-coupled receptor. Biochim Biophys Acta. 1998;1399:51–56. doi: 10.1016/s0167-4781(98)00091-8. [DOI] [PubMed] [Google Scholar]
- 14.Mayer H, Bauer H, Prohaska R. Organization and chromosomal localization of the human and mouse genes coding for LanC-like protein 1 (LANCL1) Cytogenet Cell Genet. 2001;93:100–104. doi: 10.1159/000056958. [DOI] [PubMed] [Google Scholar]
- 15.Mayer H, Bauer H, Breuss J, Ziegler S, Prohaska R. Characterization of rat LANCL1, a novel member of the lanthionine synthetase C-like protein family, highly expressed in testis and brain. Gene. 2001;269:73–80. doi: 10.1016/s0378-1119(01)00463-2. [DOI] [PubMed] [Google Scholar]
- 16.Chung CH, Kurien BT, Mehta P, Mhatre M, Mou S, Pye QN, Stewart C, West M, Williamson KS, Post J, Liu L, Wang R, Hensley K. Identification of lanthionine synthase C-like protein-1 as a prominent glutathione binding protein expressed in the mammalian central nervous system. Biochemistry. 2007;46:3262–3269. doi: 10.1021/bi061888s. [DOI] [PubMed] [Google Scholar]
- 17.Bauer H, Mayer H, Marchler-Bauer A, Salzer U, Prohaska R. Characterization of p40/GPR69A as a peripheral membrane protein related to the lantibiotic synthetase component C. Biochem Biophys Res Commun. 2000;275:69–74. doi: 10.1006/bbrc.2000.3260. [DOI] [PubMed] [Google Scholar]
- 18.Li B, Yu JP, Brunzelle JS, Moll GN, van der Donk WA, Nair SK. Structure and mechanism of the lantibiotic cyclase involved in nisin biosynthesis. Science. 2006;311:1464–1467. doi: 10.1126/science.1121422. [DOI] [PubMed] [Google Scholar]
- 19.Li B, van der Donk WA. Identification of essential catalytic residues of the cyclase NisC involved in the biosynthesis of nisin. J Biol Chem. 2007;282:21169–21175. doi: 10.1074/jbc.M701802200. [DOI] [PubMed] [Google Scholar]
- 20.Landlinger C, Salzer U, Prohaska R. Myristoylation of human LanC-like protein 2 (LanCL2) is essential for the interaction with the plasma membrane and the increase in cellular sensitivity to adriamycin. Biochim Biophys Acta. 2006;1758:1759–1767. doi: 10.1016/j.bbamem.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Blisnick T, Vincensini L, Barale JC, Namane A, Braun BC. LanCL1, an erythrocyte protein recruited to the Maurer's clefts during Plasmodium falciparum development. Mol Biochem Parasitol. 2006;141:39–47. doi: 10.1016/j.molbiopara.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Park S, James CD. Lanthionine synthetase components C-like 2 increases cellular sensitivity to adriamycin by decreasing the expression of P-glycoprotein through a transcription-mediated mechanism. Cancer Res. 2003;63:723–727. [PubMed] [Google Scholar]
- 23.Liu X, Yue Y, Li W, Ma L. Response to comment on “A G protein-coupled receptor is a plasma membrane receptor for the plant hormone abscisic acid”. Science. 2007;318:914d. doi: 10.1126/science.1135882. [DOI] [PubMed] [Google Scholar]