Abstract
Plant defenses are expected to be negatively correlated with plant growth, development and reproduction. In a recent study, we investigated the specificity of induction responses of chemical defenses in the Brassicaceae Sinapis alba.1 It was shown that glucosinolate levels and myrosinase activities increased to different degrees after 24-hours-feeding by a specialist or generalist herbivore or mechanical wounding. Here, we present the specific influences of these treatments on organ biomasses which were recorded as a measure of growth. Directly after the treatments, organ biomasses were reduced locally and systemically by herbivore feeding, but not by mechanical wounding compared to control plants. Induction of glucosinolates, which increased in all treatments, is thus not necessarily expressed as cost in terms of reduced growth in S. alba. No significant long-term differences in plant development between herbivore treated and control plants were found. Thus, tissue loss and increased investments in chemical defenses could be compensated over time, but compensation patterns depended on the inducing agent. Furthermore, herbivore treatments resulted in an increased mechanical defense, measured as abaxial trichome densities. Plants respond highly dynamic with regard to defense and growth allocation and due to different inductors.
Key words: Brassicaceae, organ biomass, plant development, specialist, generalist, herbivore, mechanical wounding, costs, trichome density
Plant defenses are generally thought to impose costs in relation to growth and fitness.2 The ability to increase defense levels only after herbivory, i.e., induction, is one possible mechanism of lowering these allocation costs.3 In Brassicaceae, the glucosinolate-myrosinase system is known to hold a defensive function.4 The constitutive and induced production of glucosinolates and myrosinases is thought to be connected to allocation and ecological costs.2,5
In a recent study, we investigated the specificity of short-term induction patterns of chemical defenses in Sinapis alba L. var. Silenda damaged by a glucosinolate-sequestering specialist herbivore (turnip sawfly, Athalia rosae (L.), Hymenoptera), a generalist herbivore (fall armyworm, Spodoptera frugiperda J. E. Smith, Lepidoptera) or mechanical wounding (cork borer).1 Feeding by the specialist as well as mechanical wounding led to 3-fold increases in both glucosinolate- and myrosinase-levels, whereas generalist feeding induced up to 2-fold increases in glucosinolates only.
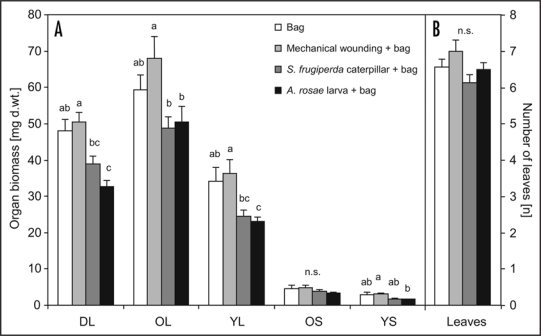
Different strengths of plant chemical responses might be mirrored in differences of subsequent fitness-related parameters of the plants.6 To assess short-term effects within 24 hours of induction on organ growth in S. alba, organ dry biomasses were calculated from the previous plant set.1 Water content was determined of the organ halves which were freeze-dried and analyzed for glucosinolate content1 and organ dry weights were calculated from water content and total organ fresh weight. The percentage of removed tissue area was determined by photo analysis and organ dry weights of treated leaves were corrected for the respective area. The percentage of lost area in damaged leaves was 7.9 ± 0.5 % after mechanical wounding, 15.1 ± 2.3 % after feeding by S. frugiperda and 15.6 ± 2.3 % after feeding by A. rosae (mean values ± SE, n = 7–8). The plants' habits and total number of leaves did not vary between the tested plant groups (Fig. 1B; ANOVA: f = 2.36, df = 3, p = 0.095).
Figure 1.
Organ dry biomasses of leaves and stems (A) and total numbers of leaves (B) of Sinapis alba cv. Silenda directly after induction. The second youngest leaves of three weeks old plants were treated with either mechanical wounding (cork borer), one Spodoptera frugiperda caterpillar (third instar) or one larva of Athalia rosae (third instar) enclosed in a muslin bag for 24 hours. Bagged leaves without any further treatment served as controls (mean values ± SE, n = 6–8 per treatment). Letters above bars indicate significant differences (ANOVA, Tukey-HSD tests: p < 0.05; n.s., not significant). DL, damaged leaf; OL, older leaf; YL, younger leaf; OS, older stem; YS, younger stem.
The short-term growth responses were highly specific between treatments. Herbivore damage did not only result in reduced organ biomass growth of the damaged leaf (ANOVA: f = 11.29, df = 3, p < 0.001), but also of adjacent tissues compared to organs from bag treated and mechanically wounded plants after 24 hours of treatment (Fig. 1A; older leaf - ANOVA: f = 3.87, df = 3, p = 0.021; younger leaf - ANOVA: f = 6.02, df = 3, p = 0.003; younger stem - ANOVA: f = 4.12, df = 3, p = 0.017). Significant differences from bag treated control plants were found for damaged and systemic younger leaves of plants treated with A. rosae larvae. Differences of organ dry biomasses between mechanically wounded and herbivore treated plants were more pronounced, with reduced growth in the latter of 15 to 36 % in leaves and 23 to 48 % in stem parts. This specificity in growth response could be brought about by elicitors introduced to the wounded plant tissues from the herbivores' saliva which can influence C-allocation to roots.7 The reduced growth of organ biomasses observed in herbivore treated leaves could be the result of specifically saliva elicited resource allocation away from leaf tissue,8 and might not represent costs of increased chemical defense.
Long-term effects of herbivore feeding on development of S. alba were monitored in a second set of plants which were treated (as described previously in ref. 1) for 24 hours with either the specialist or the generalist, enclosed in a bag. About three weeks later, on the day when the first flower opened, several parameters were recorded (Table 1). The long-term development of all three plant groups was very similar (Table 1). However, total leaf area was significantly increased in plants treated previously with specialist A. rosae feeding compared to those treated with a bag only or with generalist S. frugiperda feeding. Thus, in the long-term plants that had been attacked by herbivores did actually compensate for leaf area loss, possibly with increased growth rates. Tolerance to loss of photosynthetic tissue has been shown for several plant species.9,10 Thereby, thresholds for damage seem to exist, beyond which no compensation of tissue loss is possible.11 The percentages of damage in S. alba were, however, below the threshold values reported for other Brassicaceae.11 Influences on growth rates can be obviously transitory. In Arabidopsis thaliana (L.) Heynh., reduced growth rates were observed directly after treatment, but later growth increased so much, that these plants overcompensated and were even larger than control plants.9 Such plastic plant responses can be again modified by elicitors.7,12
Table 1.
Developmental responses of 3-week-old Sinapis alba plants treated for 24 hours with either one larva of the specialist Athalia rosae or one caterpillar of the generalist Spodoptera frugiperda
| ANOVE | Levené | ||||||
| Plant parameter | Bag | S. frugiperda + bag | A. rosae + bag | F | P | F | P |
| Number of leaves [n] | 14.20 (1.36) | 14.20 (0.49) | 14.25 (1.70) | 0.001 | 0.999 | 1.699 | 0.228 |
| Total leaf area [cm2] | 378.85 (16.96) ab | 365.01 (23.45) a | 463.52 (37.60) b1 | 4.068 | 0.048 | 2.641 | 0.116 |
| Aboveground biomass, fresh weight [g] | 19.81 (1.24) | 20.58 (0.67) | 22.37 (1.51) | 1.234 | 0.328 | 1.673 | 0.232 |
| Days to first flower[d] | 14.20 (0.58) | 14.60 (1.08) | 12.75 (0.85) | 1.161 | 0.349 | 1.400 | 0.287 |
| Number of buds [n] | 150.80 (16.23) | 148.40 (4.30) | 157.75 (21.80) | 0.099 | 0.907 | 4.453 | 0.038 |
| Trichome density, abaxial LS, treated leaf [n/cm2] | 31.28 (5.55) a | 57.71 (7.68) b | 47.91 (2.90) ab | 5.169 | 0.026 | 1.231 | 0.329 |
| Trichome density, abaxial LS, treated leaf [n/cm2] | 16.74 (3.92) | 23.35 (2.84) | 19.27 (1.88) | 1.195 | 0.339 | 1.969 | 0.186 |
| Trichome density, abaxial LS, +3 leaf [n/cm2] | 51.99 (17.90) a | 159.49 (31.15) b | 72.14 (15.48) ab | 6.156 | 0.016 | 0.780 | 0.482 |
| Trichome density, abaxial LS, +3 leaf [n/cm2] | 29.52 (11.29) | 37.01 (8.08) | 33.59 (1.05) | 0.200 | 0.822 | 6.115 | 0.016 |
Larvae were enclosed on the second-youngest leaf in a muslin bag. Leaves of control plants were enclosed in bags as well. Insects and bags were removed after the 24 hour period. Plants were harvested on the day the first flower opened (about three weeks after treatment). Mean values (SE), n = 5. Notes: 1 - multiple comparisons were marginally significant with P = 0.052. Abbreviations: LS - leaf side, +3 leaf - leaf that was three positions further up on the stem from the induction site. Treatment effects were tested by one-way ANOVA followed by HSD tests (significant differences are marked with different letters and values highlighted in bold, P < 0.05, or otherwise stated). Variance homogeneity was examined by Levené-tests.
Specific reactions of S. alba were also observed in the production of trichomes. Early herbivore feeding led to an increase of trichome densities on abaxial leaf sides in the damaged leaf, but much more pronounced in the leaf three positions further up that expanded after induction treatment (+3 leaves). Due to generalist feeding trichome densities doubled in treated and tripled in the +3 leaves, whereas the increase of trichomes due to specialist feeding was less pronounced. Investment in this mechanical defense was not mirrored in a potential reduced short-term growth, but possibly prevented generalist induced plants from overcompensation of growth in the long term.
The general trade-off between growth and defense is well known. In contrast to these long-term evolutionary associations between plant species, within individual plants initially reduced growth rates after induction treatments might be involved in a tolerance mechanism rather than an expression of costs from increased chemical or mechanical defenses. In S. alba induced chemical defenses, mechanical defenses and growth responses showed different specific patterns according to herbivore species or mechanical wounding. Putative tolerance mechanisms by increased C-allocation into root tissues7 might enable plants to cope with short-term herbivore feeding, but might depend on the herbivore's impact. As shown here, tolerance mechanisms are not, as formerly suggested, restricted as response to specialist herbivores,7 but were also observable after generalist feeding. The identification of herbivore derived elicitors, their signaling cascades and possible integration points between several defense mechanisms and growth will further aid in understanding the plasticity of plant behavior in response to signaling events.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: www.landesbioscience.com/journals/psb/article/5298
References
- 1.Travers-Martin N, Müller C. Specificity of induction responses in Sinapis alba L. and their effects on a specialist herbivore. J Chem Ecol. 2007;33:1582–1597. doi: 10.1007/s10886-007-9322-1. [DOI] [PubMed] [Google Scholar]
- 2.Strauss SY, Rudgers JA, Lau JA, Irwin RE. Direct and ecological costs of resistance to herbivory. Trends Ecol Evol. 2002;17:278–285. [Google Scholar]
- 3.Cipollini D, Purrington CB, Bergelson J. Costs of induced responses in plants. Basic Appl Ecol. 2003;4:79–85. [Google Scholar]
- 4.Halkier BA, Gershenzon J. Biology and biochemistry of glucosinolates. Annu Rev Plant Biol. 2006;57:303–333. doi: 10.1146/annurev.arplant.57.032905.105228. [DOI] [PubMed] [Google Scholar]
- 5.Siemens DH, Garner SH, Mitchell-Olds T, Callaway RM. Cost of defense in the context of plant competition: Brassica rapa may grow and defend. Ecology. 2002;83:505–517. [Google Scholar]
- 6.Soler R, Bezemer TM, van der Putten WH, Vet LEM, Harvey JA. Root herbivore effects on above-ground herbivore, parasitoid and hyperparasitoid performance via changes in plant quality. J Anim Ecol. 2005;74:1121–1130. [Google Scholar]
- 7.Schwachtje J, Minchin PEH, Jahnke S, van Dongen JT, Schittko U, Baldwin IT. SNF1-related kinases allow plants to tolerate herbivory by allocating carbon to roots. Proc Natl Acad Sci USA. 2006;103:12935–12940. doi: 10.1073/pnas.0602316103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thorpe MR, Ferrieri AP, Herth MM, Ferrieri RA. C-11-imaging: Methyl jasmonate moves in both phloem and xylem, promotes transport of jasmonate, and of photoassimilate even after proton transport is decoupled. Planta. 2007;226:541–551. doi: 10.1007/s00425-007-0503-5. [DOI] [PubMed] [Google Scholar]
- 9.Dietrich R, Ploss K, Heil M. Growth responses and fitness costs after induction of pathogen resistance depend on environmental conditions. Plant Cell Environ. 2005;28:211–222. [Google Scholar]
- 10.Rebek KA, O'Neil RJ. The effects of natural and manipulated density regimes on Alliaria petiolata survival, growth and reproduction. Weed Res. 2006;46:345–352. [Google Scholar]
- 11.Mauricio R, Bowers MD, Bazzaz FA. Pattern of leaf damage affects fitness of the annual plant Raphanus sativus (Brassicaceae) Ecology. 1993;74:2066–2071. [Google Scholar]
- 12.van Kleunen M, Ramponi G, Schmid B. Effects of herbivory simulated by clipping and jasmonic acid on Solidago canadensis. Basic Appl Ecol. 2004;5:173–181. [Google Scholar]