Abstract
Thirty-one CLAVATA3/ENDOSPERM SURROUNDING REGION (ESR)-related (CLE) proteins are encoded in the Arabidopsis genome, and they are supposed to function as dodecapeptides with two hydroxyproline residues. Twenty-six synthetic CLE peptides, corresponding to the predicted products of the 31 CLE genes, were examined in Arabidopsis and rice. Nineteen CLE peptides induced root meristem consumption, resulting in the short root phenotype in Arabidopsis and rice, whereas no CLE peptides affected the shoot apical meristem in rice. Database searches revealed 47 putative CLE genes in the rice genome. Three of the rice CLE genes, OsCLE502, OsCLE504 and OsCLE506, encode CLE proteins with multiple CLE domains, which are not found in the Arabidopsis genome, and polyproline region was found between these CLE domains. These results indicate conserved and/or diverse CLE functions in each plant species.
Key words: CLE, CLAVATA, meristem, SAM, RAM, peptide
Intercellular communication is a fundamental mechanism for coordinating the development of complex bodies of multicellular organisms such as plants and animals. Peptide signaling in plants has been largely overlooked for many years, despite the importance of peptide signaling in animals, yeast and other organisms. The recent identification of several peptide hormones indicated the importance of cell-cell communication1,2 in defense responses,3 cell proliferation,4 cell differentiation,5 shoot apical meristem (SAM) size regulation,6 self-incompatibility in crucifer species,7 and stomatal patterning.8
CLAVATA3 (CLV3), and tracheary element differentiation inhibitory factor (TDIF) were shown to be involved as CLAVATA3/ENDOSPERM SURROUNDING REGION (ESR)-related (CLE) members, and they function as dodecapeptides.5,6 Chemically biosynthesized peptides could be a powerful tool for examining the CLE peptide functions. Twenty-six putative CLE peptides encoded in the Arabidopsis genome were investigated. Nineteen CLE peptides functioned not only in Arabidopsis but also in rice to reduce root apical meristem (RAM) size, whereas no Arabidopsis peptides affected rice SAM size in our assay system. However, 10 CLE peptides exhibited a strong effect on the Arabidopsis SAM.9 This may indicate that the CLE peptides function less redundantly in the SAM than in the RAM, and that some Arabidopsis CLE peptides can bind less effectively to rice receptors due to the sequence differences between Arabidopsis and rice.
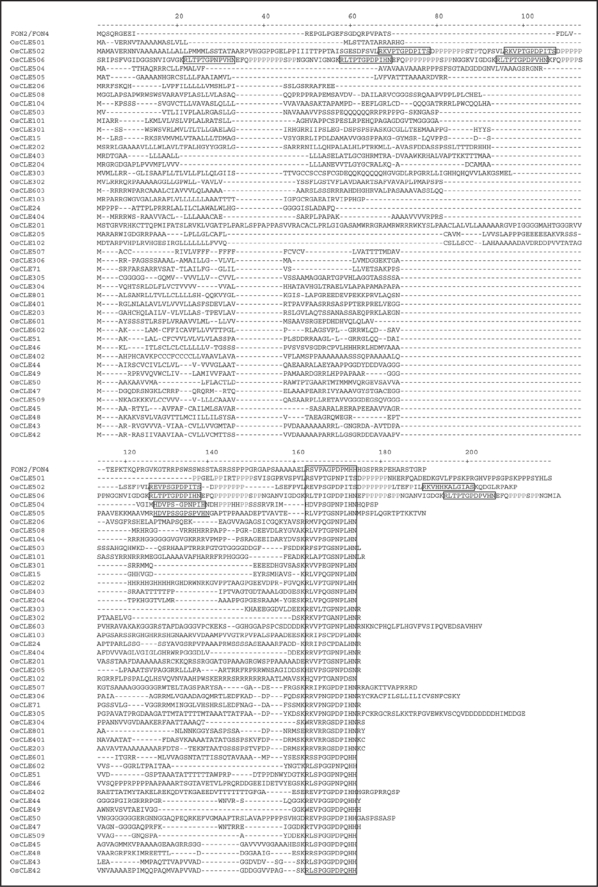
Sequencing of the rice genome has finished, and rice genes encoding putative CLE domains were database searched from RAP-DB (http://rapdb.lab.nig.ac.jp) using the Arabidopsis CLE sequences of 12 amino acid residues as queries. The resulting rice sequences were used as queries to repeat the step in an iterative manner. The search was terminated when no novel sequences were retrieved. A total of 47 putative CLE genes were found in the database search (Fig. 1).
Figure 1.
Alignment of the deduced polypeptides of the rice CLE gene family. The conserved dodecapeptide CLE region is boxed, and a CLE-related 13 amino acid sequence in OsCLE505 is underlined. Proline rich region is shown in gray. N-terminal 31 amino acid residues of CLE506, MSSISYFLVAMLLCN GFGFIVSAQVVGGGSS, are not shown because of limited space.
The coding regions of CLV3 and CLE40 genes are interrupted by two introns in Arabidopsis,10 and the application of these synthetic peptides to wild type plants induced SAM consumption. In rice, nine CLE genes, OsCLE201, OsCLE305, OsCLE402, OsCLE502, OsCLE506, OsCLE507, OsCLE47, OsCLE603 and FON2/FON4, have multiple exons. FON2/FON4 has been reported to regulate floral meristem size in a similar manner to the CLV3 in Arabidopsis,11,12 and another eight CLE genes might be involved in meristem size regulation.
Three rice CLE genes, OsCLE502, OsCLE504 and OsCLE506, encode CLE proteins carrying multiple CLE domains (Fig. 1), although no Arabidopsis gene encodes such a CLE protein. In addition to the results described in Kinoshita et al.,9 four more putative CLE domains, OsCLE502C (REVPSGPDPITS), OsCLE502D (REVPSGPDPITS), OsCLE502E (RKVHHKALGIAS) and OsCLE506F (RLTPIGPDPIHN), were found in these rice CLEs. The two sequence-related rice CLE genes, CLE504 and CLE505, are located at interval of no more than about 2 kb on chromosome 5, suggesting that they have arisen by local gene duplication events. However, OsCLE504 has two putative CLE domains, whereas OsCLE505 has one apparent CLE domain and another CLE-like motif composed of an unusual 13 amino acid residues, HDVPSSGPSPVHN, which should correspond to OsCLE504A (Fig. 1). The CLE-like peptide might not function any longer as a CLE peptide due to an additional amino acid. It is possible that these sister CLE genes might acquire different functions during their evolutionary steps. OsCLE506 encodes six CLE domains and it might function in rice-specific physiological events, but not in morphological events, because the transgenic plants harboring the RNAi construct of the OsCLE506 gene did not show any obvious morphological abnormalities (Sawa et al., unpublished results).
Most of the conserved CLE domains were located at or near the C-terminal end of the sequences we identified, but every CLE domain located at the middle region of the OsCLE502, OsCLE504 and OsCLE506 proteins was followed by an acidic amino acid residue and a characteristic polyproline region (Fig. 1). The polyproline sequence often adopts a rigid, rod-like secondary structure called as a polyproline type II helix, which is capable of serving as a protein-protein interaction domain or organizing unfolded polypeptides.13,14 In the case of the yeast peptide pheromone, alpha factor, four copies of 13 amino acid peptides are produced from one precursor.15 The KEX2-encoded endoprotease cleaves the precursor after pairs of basic residues such as lysine and arginine and these basic residues are chopped out by a carboxypeptidase, KEX1.13 Thus, CLE proteins with multiple CLE domains might be precursors and require a similar maturation steps. In this context, the polyproline region might serve as or provide a recognition site for the first endoprotease. Many CLE precursors have lysine and/or arginine residues just after the CLE domain, and this also supports the idea that carboxypeptidases are responsible for the C-terminal maturation step.
CLE proteins are currently one of the best described families of small polypeptides in plants; however, precise molecular details, such as CLE peptide maturation, movement, reception and signaling in a target cell, remain to be solved. Further genetic and biochemical analyses of the CLE family would give insights to help unveil not only the molecular mechanisms, but also the diversity and evolution of intercellular communication in plants.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/5344
References
- 1.Sawa S, Kinoshita A, Nakanomyo I, Fukuda H. CLV3/ESR-related (CLE) Peptides as Intercellular Signaling Molecules in Plant. Chem Rec. 2006;6:303–310. doi: 10.1002/tcr.20091. [DOI] [PubMed] [Google Scholar]
- 2.Fukuda H, Hirakawa Y, Sawa S. Peptide signaling in vascular development. Curr Opin Plant Biol. 2007;10:477–482. doi: 10.1016/j.pbi.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Pearce G, Moura DS, Stratmann J, Ryan CA. Production of multiple plant hormones from a single polyprotein precursor. Nature. 2001;411:817–820. doi: 10.1038/35081107. [DOI] [PubMed] [Google Scholar]
- 4.Matsubayashi Y, Sakagami Y. Phytosulfokine, sulfated peptides that induce the proliferation of single mesophyll cells of Asparagus officinalis L. Proc Natl Acad Sci USA. 1996;93:7623–7627. doi: 10.1073/pnas.93.15.7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito Y, Nakanomyo I, Motose H, Iwamoto K, Sawa S, Dohmae N, Fukuda H. Dodeca-CLE peptides as suppressors of plant stem cell. Science. 2006;313:842–845. doi: 10.1126/science.1128436. [DOI] [PubMed] [Google Scholar]
- 6.Kondo T, Sawa S, Kinoshita A, Mizuno S, Kakimoto T, Fukuda H, Sakagami Y. A plant peptide encoded by CLV3 identified by in situ MALDI TOF-MS analysis. Science. 2006;313:845–848. doi: 10.1126/science.1128439. [DOI] [PubMed] [Google Scholar]
- 7.Schopfer CR, Nasrallah ME, Nasrallah JB. The male determinant of self-incompatibility in Brassica. Science. 1999;286:1697–1700. doi: 10.1126/science.286.5445.1697. [DOI] [PubMed] [Google Scholar]
- 8.Hara K, Kajita R, Torii KU, Bergmann DC, Kakimoto T. The secretory peptide gene EPF1 enforces the stomatal one-cell-spacing rule. Genes Dev. 2007;21:1720–1725. doi: 10.1101/gad.1550707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinoshita A, Nakamura Y, Sasaki E, Kyozuka J, Fukuda H, Sawa S. Gain-of-function phenotypes of chemically synthetic CLAVATA3/ESR-related (CLE) peptides in Arabidopsis thaliana and Oryza sativa. Plant Cell Physiol. 2007;48:1821–1825. doi: 10.1093/pcp/pcm154. [DOI] [PubMed] [Google Scholar]
- 10.Hobe M, Muller R, Grunewald M, Brand U, Simon R. Loss of CLE40, a protein functionally equivalent to the stem cell restricting signal CLV3, enhances root waving in Arabidopsis. Dev Genes Evol. 2003;213:371–381. doi: 10.1007/s00427-003-0329-5. [DOI] [PubMed] [Google Scholar]
- 11.Suzaki T, Toriba T, Fujimoto M, Tsutsumi N, Kitano H, Hirano HY. Conservation and diversification of meristem maintenance mechanism in Oryza sativa: Function of the FLORAL ORGAN NUMBER2 gene. Plant Cell Physiol. 2006;47:1591–1602. doi: 10.1093/pcp/pcl025. [DOI] [PubMed] [Google Scholar]
- 12.Chu H, Qian Q, Liang W, Yin C, Tan H, Yao X, Yuan Z, Yang J, Huang H, Luo D, Ma H, Zhang D. The floral organ number4 gene encoding a putative ortholog of Arabidopsis CLAVATA3 regulates apical meristem size in rice. Plant Physiol. 2006;142:1039–1052. doi: 10.1104/pp.106.086736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dmochowska A, Dignard D, Henning D, Thomas D Y, Bussey H. Yeast KEX1 gene encodes a putative protease with a carboxypeptidase B-like function involved in killer toxin and alpha factor precursor processing. Cell. 1987;50:573–584. doi: 10.1016/0092-8674(87)90030-4. [DOI] [PubMed] [Google Scholar]
- 14.Rath A, Davidson AR, Deber CM. The structure of “unstructured” regions in peptides and proteins: role of the polyproline II helix in protein folding and recognition. Biopolymers. 2005;80:179–185. doi: 10.1002/bip.20227. [DOI] [PubMed] [Google Scholar]
- 15.Kay BK, Williamson MP, Sudol M. The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 2000;14:231–241. [PubMed] [Google Scholar]